Department of Auditory Neuroscience
 Head: Prof. MUDr. Josef Syka, DrSc.
Head: Prof. MUDr. Josef Syka, DrSc.
Scientists:
MUDr. Daniela Buckiova, CSc.
Ing. Milan Jilek
RNDr. Jiri Popelar, CSc.
RNDr. Natalia Rybalko, CSc.
Dr. Ing. Daniel Suta
RNDr. Rostislav Turecek, Ph.D.
Ph.D. Students:
Mgr. Bohdana Hruskova
MUDr. Ladislav Ouda
MUDr. Jana Pelanova
Mgr. Johana Trojanova
Technical Assistants:
Jana Janouskova
Jan Kolar
Jan Setnicka
Eva Velebna
Address:
Videnska 1083, 142 20 Praha 4
Phone: (+420) 296 442 700
Fax: (+420) 296 442 787
E-mail: syka@biomed.cas.cz
The main research aim in the Department of Auditory Neuroscience is to investigate the structure
and function of the auditory system in animal models under normal conditions and also under different pathological situations such as noise exposure, the
administration of ototoxic drugs and stimulation of the auditory system by electrical current. Selected problems are also investigated in man, either in volunteer
subjects or in subjects with auditory system pathologies. The experimental approaches are used to solve practical clinical problems such as the diagnosis and
treatment of perception deafness and the development of cochlear neuroprostheses. The methods employed range from anatomical and histochemical
techniques (tracing of neural tracts by HRP and neurodegenerative techniques, histochemistry of cytochrome oxidase and NADPH-diaphorase,
immunocytochemistry of calbindin, parvalbumin and GAD) to electrophysiological techniques (intracellular recording, patch clamp, extracellular recording of
single unit activity, recording of evoked potentials with implanted electrodes, recording of DC potentials), behavioral techniques (operant conditioning
procedures for estimating hearing functions in laboratory animals) and special audiological and psychoacoustic methods such as the measurement of otoacoustic
emissions, the estimation of difference limens for frequency and intensity, and gap function. In addition, methods of nuclear magnetic resonance such as
functional magnetic resonance imaging are used in collaboration with the Department of Nuclear Magnetic Resonance of the Institute of Clinical and Experimental
Medicine. Studies in experimental audiology in patients are performed in collaboration with the ENT Clinics of the 1st and 2nd Medical Faculties of Charles
University in Prague
Current research at the Department of Auditory Neuroscience focuses on the following topics:
- Morphology of the central auditory pathway
- Physiology and development of the GABA and glycinergic systems in the auditory nuclei
- The role of subcortical auditory nuclei and the auditory cortex in the processing of acoustical signals
- The role of descending pathways in the auditory system
- Changes in the auditory system in pathological situations
- Estimation of hearing function in experimental animals using behavioral techniques
- Studies of hearing function in man
Morphology of the central auditory pathway
Progress has been made in describing the descending corticotectal auditory pathway. In the rat,
descending fibers are axons of the layer V pyramidal cells, and the corticogeniculate pathway originates in layer VI. The descending fibers originate in all three
main auditory areas. NADPH-diaphorase-positive neurons are present in the auditory pathway of the rat in subnuclei, which belong to secondary parts of the
auditory pathway, i.e. in the external and dorsal cortices of the inferior colliculus and in the medial and suprageniculate nucleus of the medial geniculate
body. In the cortex they are dispersed in all layers with the exception of layers I and III. Bilateral ablation of the auditory cortex in rats results
in a temporary decrease of NADPH-diaphorase staining in the inferior colliculus. Complementary to the distribution of NADPH-diaphorase, cytochromoxidase
has been found in the primary parts of the auditory pathway both in rats and in guinea pigs. A similar complementary distribution was found for the calcium
binding proteins calbindin and parvalbumin. For example, in cochlear nuclei calbindin is present in the dorsal cochlear nucleus whereas parvalbumin-positive
cells are located in the anteroventral nucleus. Large changes in the thickness of the auditory and visual cortices were observed in very old rats
(more than three years old) accompanied by changes in the NADPH-diaphorase neurons. A decrease in the number of NADPH-diaphorase-positive neurons in the
inferior colliculus of the rat was described several days after auditory cortex ablation. At the present time the distribution of glutamic-acid
decarboxylase (GAD) in the auditory cortex of rats exposed to noise is a subject of study.
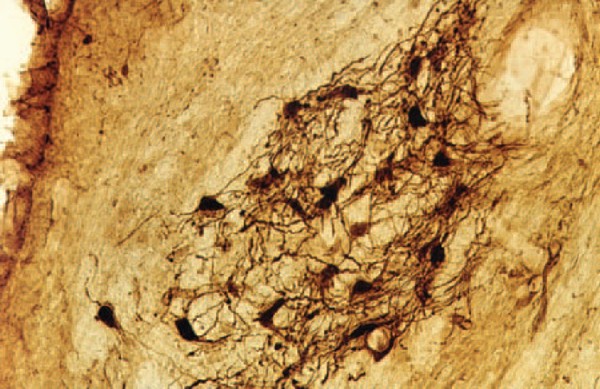
Fig. 1: Calbindin- positive octopus cells in the posteroventral cochlear nucleus of rat
Physiology and development of GABA and glycinergic systems in the auditory nuclei
The goal of our current projects has been to reveal the mechanisms modulating glutamate release from giant nerve terminals (calyces of Held) in the rat
medial nucleus of the trapezoid body (MNTB). Principal cells of the MNTB play a key role in the acoustic information processing by the brain stem auditory circuitry.
These cells accurately convert excitatory signals provided by the ventral cochlear nucleus to inhibitory signals directed to the lateral/medial superior olive. Our
studies using presynaptic patch-clamp recording provided evidence for the presence of glycine and GABAA receptors in the calyx of Held. These receptors
activated by GABA and glycine spillover from the inhibitory fiber endings induced a strongly facilitated glutamate release from the calyx. The mechanism underlying the
facilitation appeared to involve a weak depolarization of a nerve terminal, an activation of presynaptic voltage-gated calcium channels, and presynaptic Ca2+
accumulation. We found that in animals younger than postnatal day 11 (P11), and prior to the onset of hearing, the terminals expressed mostly GABAA receptors.
After P11, GABAA receptors were largely absent from the terminals and replaced by glycine receptors.
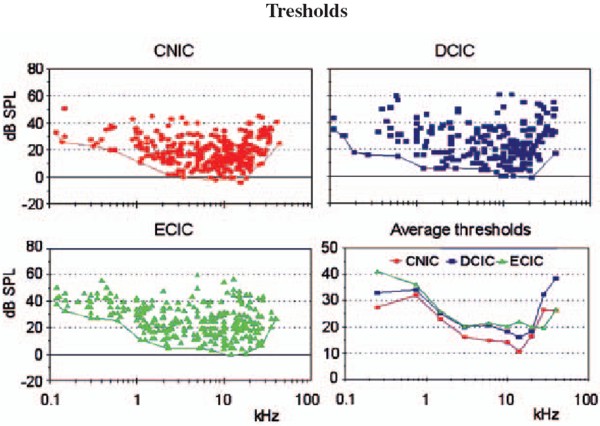
Fig. 2: Mapping of neuronal thresholds to sound stimuli in the inferior colliculus (IC) in guinea pig.
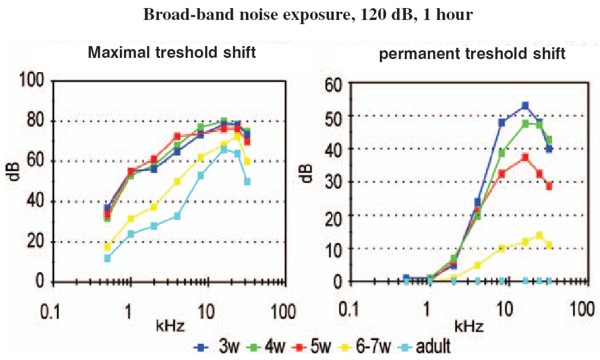
Fig. 3: Threshold shifts after one hour exposure of broad-band noise 120 dB SPL in young and adult rats
The role of subcortical auditory nuclei in the processing of acoustical signals
Mapping of the parameters of neuronal responses in the inferior colliculus (IC) in guinea pig revealed the tonotopical
organisation of this nucleus, which is similar to that in other mammalian species. Isofrequency layers spread through all three subnuclei of the IC; however,
the responses of neurons in the "secondary" parts of the IC are different in some parameters from those in the "primary" parts (the sharpness of tuning is smaller,
the latencies of responses are longer and the average thresholds are higher). Differences between the secondary and primary parts were also found in the responses
of IC neurons to species-specific vocalizations, particularly in cats. However, a high degree of specificity does not exist in the reactions of IC neurons in the
guinea pig to vocalizations of its own species. Many of the IC neurons in the guinea pig react to all types of animal vocalizations as well as to noise and pure tones.
Similarly, in the medial geniculate body of the guinea pig we did not find specific reactions to species-specific vocalizations, although neurons in the medial geniculate
body prefer to respond to the beginning of acoustical stimuli, including vocalizations. Both in the IC and MGB, the spectrotemporal acoustic pattern of vocalization
sounds is encoded in a distributed way by a population of neurons.
The role of descending pathways in the auditory system
One of the longstanding topics in the department is the study of the structure and function of the descending pathways in the
central auditory system. After performing several morphological studies, a technique of functional ablation of the auditory cortex was introduced to investigate the role
of the corticotectal pathway descending from the layer five pyramidal neurons to the inferior colliculi, mostly on the ipsilateral side. The application of the sodium
channel blocker tetrodotoxin on the surface of the auditory cortex resulted in the prolongation of the latencies of evoked potentials in the IC, in increases or
decreases of the neuronal responses to sound in the IC, as well as in changes in the functional interaction in pairs of IC neurons recorded with one microelectrode.
Another study demonstrated that electrical stimulation of distinct loci in the IC produces changes in the distortion product otoacoustic emissions on the ipsilateral
side in the guinea pig.
Changes in the auditory system in pathological situations
The exposure of awake, non-anaesthetised guinea pigs to noise results in an enhancement of middle latency responses (MLR) to
acoustical stimuli. The amplitude of MLR enhancement is related to the intensity and duration of the noise exposure. The results suggest that the post-exposure amplitude
enhancement may be caused by a temporary exhaustion of inhibitory processes in the auditory cortex. Similar effects of noise exposure were observed in rats - a
species with typical high frequency hearing. High intensity noise exposure in rats also results in temporary threshold shifts accompanied by enhanced MLR.
Recovery functions of both processes (i.e., the threshold shift and the amplitude enhancement) are different, which supports the idea that threshold shifts are
generated in the inner ear whereas amplitude enhancement is caused by processes in the auditory cortex or in subcortical nuclei. The effects of noise exposure are even
more pronounced in young rats during first five postnatal weeks. Noise exposure, which in adult rats produces a temporary threshold shift, results in young rats (up to
the age of 6-7 weeks) in a permanent threshold shift. Histochemical methods also indicate changes in the function of the auditory system after noise exposure.
For example, the occurrence of calbindin-positive neurons in dorsal cochlear nuclei increases immediately after noise exposure in rats.
Estimation of hearing function in experimental animals with behavioral techniques
Operant conditiong procedures are used to investigate hearing function in rats, with the aim of comparing the results with
the results of psychoacoustical tests in man or with the results of audiological tests in patients. Hearing sensitivity in rats was found to range from 0.5 kHz
to 64 kHz, with the lowest threshold values present between 10 and 30 kHz. Frequency discrimination in rats was found to be substantially poorer than in man or in the
majority of mammalian species so far tested, with Weber ratios (frequency difference limen divided by frequency) between 3.7 and 7.6%. The Weber ratio in man is around
0.3%. Intensity difference limens were frequency independent and amounted to 2.9 ± 0.5 dB in conditions of intensity increases and 6.5 ± 1.6 dB in conditions of
intensity decreases. Temporal resolution in rats was measured by detection of gaps in a continuosly presented sound (gap function). Thresholds for gap detection were
found to be in the range of 2-3 ms, which is comparable with values found in man. In rats, only very small changes in gap detection threshold were observed after
bilateral ablation of the auditory cortex, which contrasts with the widely accepted hypothesis that the auditory cortex plays an important role in gap detection.
Studies of hearing function in man
In young volunteers exposed to 85 dB SPL white noise or music of the same intensity, greater activation of the temporal lobes was observed with the musical
signal. The laterality of auditory cortex activation by music was significantly different when the volunteers listened to either instrumental music or the same music
with the addition of a singer´s voice. Temporary threshold shifts and a significant decline in transient, distortion product and spontaneous otoacoustic emissions were
observed in young volunteers exposed for four hours to amplified music (97 dB SPL /A/) previously recorded in a discotheque. The hearing abilities of a group of
elderly subjects were compared with a group of young normal hearing volunteers. The results support the view that presbycusis in man is produced by a combination of
declining function of the auditory periphery and deteriorated function of the central auditory system.
Relevant publications before 1998
1. Johnstone, B.M., Patuzzi, R., Syka, J., Sykova, E. (1989)
Stimulus-related potassium changes in the organ
of Corti of guinea pig. J. Physiol. (London) 408: 77-92
2. Syka, J. (1989)
Experimental models of sensorineural hearing loss. Effects of noise and ototoxic drugs on
hearing. Prog. Sensory Physiol. 9: 97-170
3. Schrott, A., Melichar, I., Popelar, J., Syka, J. (1990)
Deterioration of hearing function in mice with neural
crest defect. Hearing Res. 46: 1-8
4. Taudy, M., Syka, J., Popelar, J, Ulehlova,L. (1992)
Carboplatin and cisplatin ototoxicity in guinea pig.
Audiology 31, 293-299
5. Ernst, A., Syka, J., Mest, H-J. (1992)
Electrophysiological responses of the cochlea to transient asphyxia
are influenced by arachidonate metabolites. Prostaglandins 43: 331-338
6. Popelar, J., Syka, J. (1993)
Middle latency responses to electrical stimulation of the auditory nerve in
unanaesthetised guinea pigs. Hearing Res. 67: 69-74
7. McAnally, K.I., Clark, G.M., Syka, J. (1993)
Hair cell mediated responses of the auditory nerve to electrical
stimulation of the cochlea in the cat. I. Sinusoidal stimulation. Hearing Res. 67: 55-68
8. Druga, R., Syka, J. (1993)
NADPH-diaphorase activity in the central auditory structures of the rat.
NeuroReport 4: 999-1002
9. Syka, J., Popelar, J. (1994)
Modulation of thresholds to acoustical and electrical stimulation of the intact
ear in guinea pig by furosemide and noise. Hearing Res. 75: 1-10
10. Syka, J., Rybalko, N., Popelar, J. (1994)
Enhancement of the auditory cortex evoked responses in awake
guinea pigs after noise exposure. Hearing Res. 78: 158-168
11. Aitkin, L., Tran, L., Syka, J. (1994)
The responses of neurons in subdivisions of the inferior colliculus of cats
to tonal, noise and vocal stimuli. Exp. Brain Res. 98: 53-64
12. Popelar, J., Hartmann, R., Syka, J., Klinke, R. (1995)
Middle latency responses to acoustical and electrical
stimulation of the cochlea in cats. Hearing Res. 92: 63-77
13. Syka, J., Rybalko, N., Brozek, G., Jilek, M. (1996)
Auditory frequency and intensity discrimination in
pigmented rats. Hearing Res. 100: 107-113
14. Astl, J., Popelar, J., Kvasnak, E., Syka, J. (1996)
Comparison of response properties of neurons in the
inferior colliculus of guinea pig under different anesthetics. Audiology 35: 335-345
15. Druga, R., Syka, J., Rajkowska, G. (1997)
Projections of auditory cortex onto the inferior colliculus in the
rat. Physiol. Res. 46: 215-222
Publications 1998-2004
1. Syka, J., Popelar, J., Kvasnak, E., Suta, D. (1998)
Processing of vocalization signals in neurons of the infe-
rior colliculus and medial geniculate body. In: Central Auditory Processing and Neural Modeling. (Eds.
Poon P.W. and Brugge, J.F.) Plenum Press, New York, pp. 1-11
2. Syka, J., Popelar, J., Kvasnak, E., Suta, D. (1998)
Processing of simple and complex acoustical signals in
the inferior colliculus and medial geniculate body of the guinea pig. In: Psychophysical and Physiological
Advances in Hearing. (Eds. A.R. Palmer, A. Rees, A.Q. Summerfield, R. Meddis) Whurr Publishers,
London, pp. 491-496
3. Popelar, J., Valvoda, J., Syka, J. (1999)
Acoustically and electrically evoked contralateral suppression of
otoacoustic emissions in guinea pigs. Hearing Res. 135: 61-70
4. Syka, J., Rybalko, N. (2000)
Threshold shifts and enhancement of cortical evoked responses after noise
exposure in rats. Hearing Res. 139: 59-68
5. Kvasnak, E., Suta, D., Popelar, J., Syka, J. (2000)
Neuronal connections in the medial geniculate body of
the guinea pig. Exp. Brain Res. 132: 87-102
6. Kvasnak, E., Popelar, J., Syka, J. (2000)
Discharge properties of neurones in subdivisions of the medial
geniculate body of the guinea pig. Physiol. Res. 49: 369-378
7. Syka, J., Popelar, J., Kvasnak, E., Astl, J. (2000)
Response properties of neurons in the central nucleus
and external and dorsal cortices of the inferior colliculus in guinea pig. Exp. Brain Res. 133: 254-266
8. Turecek, R., Trussell, L.O. (2000)
Control of synaptic depression by glutamate transporters. J. Neurosci.
20: 2054-2063
9. Turecek, R., Trussell, L.O. (2001)
Presynaptic glycine receptors enhance transmitter release at a mam-
malian central synapse. Nature 411: 587-590
10. Popelar, J., Erre, J.-P., Syka, J., Aran, J.-M. (2001)
Effects of contralateral acoustical stimulation on three
measures of cochlear function in the guinea pig. Hearing Res. 152: 128-138
11. Rybalko, N., Syka, J. (2001)
Susceptibility to noise exposure during postnatal development in rats. Hearing
Res. 155: 32-40
12. Druga, R., Syka, J. (2001)
Effect of auditory cortex lesions on NADPH-diaphorase staining in the inferior
colliculus of rat. NeuroReport 12: 1555-1559
13. Mazelova, J., Valvoda, J., Popelar, J., Syka, J. (2001)
The influence of single exposure to amplified music
on hearing of young listeners. In: Noise Induced Hearing Loss: Basic Mechanisms, Prevention and
Control. D.Henderson, D.Prasher, R.Kopke, R.Salvi and R.Hamernik (Eds.). NRN Publications, London, pp.
365-375
14. Syka, J. (2002)
Plastic changes in the central auditory system after hearing loss, restoration of function and
learning. Physiol. Rev. 82: 601-636
15. Turecek, R., Trussell, L.O. (2002)
Reciprocal developmental regulation of presynaptic ionotropic receptors.
Proc. Natl. Acad. Sci. USA 99: 13884-13889
16. Popelar, J., Mazelova, J., Syka, J. (2002)
Effects of electrical stimulation of the inferior colliculus on 2f1-f2
distortion product otoacoustic emissions in anesthetized guinea pigs. Hearing Res. 170: 116-126
17. Syka, J., Rybalko, N., Mazelova, J., Druga, R. (2002)
Gap detection threshold in the rat before and after
auditory cortex ablation. Hearing Res. 172: 151-159
18. Nwabueze-Ogbo, F.C., Popelar, J., Syka, J. (2002)
Changes in the acoustically evoked activity in the inferi-
or colliculus of the rat after functional ablation of the auditory cortex. Physiol. Res. 51: S95-S104
19. Mazelova, J., Popelar, J., Syka, J. (2003)
Auditory function in presbycusis: peripheral vs. central changes.
Exp. Gerontol. 38: 87-94
20. Popelar, J., Nwabueze-Ogbo, F.C., Syka, J. (2003)
Changes in neuronal activity of the inferior colliculus
in rat after temporal inactivation of the auditory cortex. Physiol. Res. 52: 615-628
21. Ouda, L., Nwabueze-Ogbo, F.C., Druga, R., Syka, J. (2003)
NADPH-diaphorase - positive neurons in the auditory cortex
of young and old rats. NeuroReport 14: 363-366
22. Suta, D., Kvasnak, E., Popelar, J., Syka, J. (2003)
Representation of species-specific vocalizations in the inferior
colliculus of the guinea pig. J. Neurophysiol. 90: 3794-3808
23. Popelar, J., Groh, D., Mazelova, J., Syka, J. (2003)
Cochlear function in young and adult Fischer
F344 rats. Hear. Res. 186: 75-84
24. Glogarova, K., Buckiova, D. (2004)
Changes in sialylation in homozygous Sp2H mouse mutant embryos. Birth Defects Res Part
A Clin Mol Teratol. 70: 142-52
25. Kyselova, V., Peknicova, J., Boubelik, M., Buckiova, D. (2004)
Body and organ weight, sperm acrosomal status and reproduction
after genistein and diethylstilbestrol treatment of CD1 mice in a multigenerational study. Theriogenology 61: 1307-1325
26. Awatramani, G.B., Turecek, R., Trussell, L.O. (2004)
Inhibitory control at a synaptic relay. J Neurosci. 24: 2643-2647
27. Turecek, R., Vlcek, K., Petrovic, M., Horak, M., Vlachova, V., Vyklický, L.Jr. (in press)
Intracellular spermine decreases
open probability of N-methyl-D-aspartate receptor channels. Neuroscience.
|