Department of Molecular Pharmacology
 Head: MUDr. Jaroslav Blahos, Ph.D.
Head: MUDr. Jaroslav Blahos, Ph.D.
Scientist:
Ing. Michaela Havlickova
Ph.D. Student:
Mgr. Veronika Blahoutova
Technical Assistants:
Daniela Frankova
Bohdana Hruskova
Johana Trojanova
Alice Zikova
Address:
Videnska 1083, 142 20 Praha 4
Phone: (+420) 296 442 725
Fax: (+420) 296 442 782
e-mail: blahos@biomed.cas.cz
Neurotransmitters typically activate several types of receptors. Glutamate binds to ligand-gated ion
channels (NMDA, AMPA, Kainate receptors) or G-protein coupled receptors (GPCRs): the metabotropic glutamate receptors (mGluRs). The inhibitory
neurotransmitter -aminobutyric acid (GABA) activates ionotropic GABAA and GABAC and metabotropic GABAB receptors. While the ionotropic
receptors are engaged in fast interneuronal transmission, the metabotropic receptors modulate neurotransmission. They are located on the pre- or postsynaptical
membranes of neurons. Such a location allows these metabotropic receptors to influence the functional properties of the synapses. Thus, metabotropic receptors
are targets for the design of new drugs and therapeutics for certain neurological disorders.
Current projects focus on the following topics:
- Structure-function relationships of the metabotropic glutamate (mGlu) and GABAB receptors
- Molecular dissection of mGlu and GABAB receptor interaction with G-proteins
- Function of proteins associated with GABAB receptor subunits
Structure-function relationships of the metabotropic glutamate (mGlu) and GABAB receptors
The development of new compounds acting on the receptors depends on our knowledge of their structure
and function. Metabotropic glutamate and GABAB receptors have a similar structure in which two proteins form a receptor complex. Metabotropic glutamate
receptors are homomeric dimers while GABAB receptor is a heterodimer composed of two proteins called GB1 and GB2. These receptors belong to "Family 3
GPCR" with common structural and functional features. The interaction of agonists with the extracellular N-terminal portion of the receptors evokes changes in
their conformation leading to the activation of the receptors. This conformational change is transmitted to the heptahelical transmembrane domains as a
change of relative position of the intracellular portions of the receptors. The intracellular portions of the heptahelical domains mediate coupling to G-proteins. Our
current research is aimed at investigating signalling mechanisms of the receptor complexes with an emphasis on mapping the molecular determinants that
modulate the activation process. Newly designed drugs acting allosterically are one of the tools that we use in our research. Describing the mechanism of their
positive or negative modulation of the receptors is done using site-directed mutagenesis, heterologous expression of the clones in mammalian cell lines with
subsequent functional tests. Biochemistry and immunohistochemistry are tools used mostly as controls for the proper expression of the proteins.
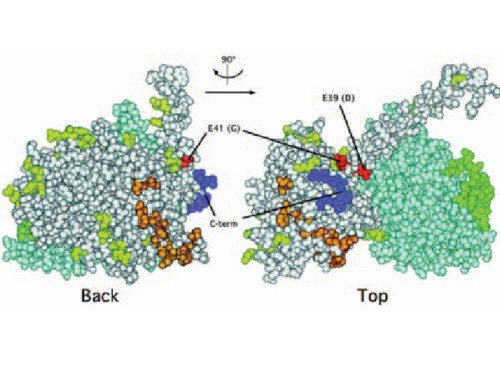
Fig 1: 3D Model of the G - protein molecule is presented in white with the 5 extreme C-terminal residues important for coupling in
dark blue. In red are residues we described as a novel site on G - protein that discriminates between mGlu receptors.
The dimeric structure of the mGlu and GABAB receptors is also studied from the point of G-protein interaction with the
heptahelical region of each protomer (that is each protein from the receptor complex). In a recent study we showed that a single heptahelical domain of one subunit
(GB2) of the GABAB receptor contains all determinants for G-protein activation. The dimeric structure of the GABAB receptor is necessary for
the activation process, but in this dimeric
structure, once activated each subunit probably may act as an independent unit or even has another role in signalling. A recently proposed mechanism of activation of the
metabotropic glutamate receptors would be in favor of such a notion. This model, based on the crystal structure of the open and closed states of the extracellular
domains of the mGluRs, suggests that the relative position of the two subunits' heptahelical domains corresponds to the active or inactive state. Our data
showing the GB2 subunit being capable of G-protein activation add new information to the spectrum of findings that are necessary for solving the puzzle
connected with GPCR activation and transmission of this activation on G-proteins. Recently, we and other groups pointed at the GB2 subunit of the heteromeric complex
as the subunit that activates G-proteins. The functional significance of the GB1 subunit transmembrane region is explored in our laboratory.
Our ongoing collaborations with the laboratory of Jean Phillipe Pin and Laurent Fagni from the C.N.R.S. unit in Montpellier,
headed by Joel Bockaert, and that of Bernhard Bettler at the University of Basel are critical for these projects.

Fig 2.: The Heterodimeric structure of GABAB receptor. Experiments with chimeric receptors proved that single heptahelical
domain (HD of the GB2 subunit) activates G-proteins. Symbol G indicates subunit that is capable of activating G-proteins; symbol x is where mutation
within intracellular portion of GB2 HD was done in order to disrupt coupling to G proteins. a) wild type receptor; b,c,d) chimerical and
mutated receptors.
Molecular dissection of mGluRs and GABAB receptor interaction with G-proteins
Each receptor activates a specific subset of G-proteins. This interaction encodes which intracellular pathway will
be modulated. Regions on the G proteins that are compatible with sequences at the receptors come into close proximity during the activation of the receptor and
transmission of this activation on the G-proteins. This compatibility is decisive for G-protein selectivity of the receptors. We are mapping the contact sites between the
receptors and G-proteins. Recently the extreme C-terminus of G -protein was shown to interact with the intracellular portions of mGluRs, specifically with the second
intracellular loop of the heptahelical transmembrane domain. This is one of the most important contact points between these proteins. Other regions on the G-proteins
are involved in the selectivity, and the partner sequences on the receptors will be described in detail.
Function of proteins associated with GABAB receptor subunits
A yeast two-hybrid system is employed in order to identify proteins that interact with the GABAB
receptor. Interactions of regulatory or transport proteins with the receptor GABAB complexes and relationships with the associated and signalling proteins are
examined using recombinant proteins expressed in heterologous cells or in neuronal cultures. Biochemical, immunochemical and molecular biology tools are used
to describe the interactions and the functional significance of these interactions. This project is carried out in collaboration with the laboratory of Dr. Pavel
Osten at the Max-Planck Institute in Heidelberg.
Relevant publications before 1998
1. Blahos, J., Whalin, M., Krueger, K.E. (1995)
Identification of a 10kD Protein Associated with Mitochondrial
Benzodiazepine Receptors. J. Biol. Chem. 270: 20285-20291
2. Wenthold, R. J., Petralia, R.S., Blahos, J., Niedzelsky, A.S. (1996)
Evidence for Multiple AMPA Receptor
Complexes in Hoppocampal CA1/CA2 Neurons. J. Neurosci. 16: 1982-1989
3. Blahos, J., Wenthold, R.J. (1996)
Relationship between NMDA Receptor NR1 Splice Variants and NR2
Subunits. J. Biol. Chem. 271: 15669-15674
Publications 1998-2002
1. Blahos, J., Mary, S., Perroy, J., de Colle, C., Brabet, I., Bockaert, J., Pin, J.-P. (1998)
Extreme C-terminus
of G-protein a-subunits contains a site thatdiscriminates between Gi-coupled metabotropic glutamate receptors. J. Biol. Chem. 273: 25765-25769
2. Wenthold, R.J., Blahos, J., Huh, K.-H., Petralia, R.S. (1999)
Detergent Solubilization and
Immunoprecipitation of Native NMDA Receptors Methods. Mol. Biol. 128: 113-9
3. Franek, M., Pagano, A., Kaupmann, K., Bettler, B., Pin, J.-P., Blahos, J. (2000)
The heteromeric GABAB
receptor recognizes G-protein a subunit C-termini. Neuropharmacology 38: 1657-1666
4. Sans, N., Petralia, R.S., Wang, Y.-X., Blahos, J., Hell, J.W., Wenthold, R.J. (2000)
A Developmental Change
in NMDA Receptor-Associated Proteins at Hippocampal Synapses. J. Neurosci. 20: 1260-1271
5. Galvez, T., Prezeau, L., Milioti, G., Franek, M., Joly, C., Froestl, W., Bettler, B., Bertrand,H.-O., Blahos, J.,
Pin, J.-P. (2000)
Mapping the Agonist Binding Site of GABAB Type 1 subunit sheds light on the activation
process of GABAB receptors. J. Biol. Chem. 275: 41166-41174
6. Blahos, J., Fischer, T., Brabet, I., Stauffer, D., Rovelli, G., Bockaert, J., Pin, J.-P. (2001)
A Novel Site on the
G-protein that Recognizes Heptahelical Receptors. J. Biol. Chem. 276: 3262-3269
7. Pagano, A., Rovelli, G., Mosbacher, J., Lohmann, T., Duthey, B., Stauffer, D., Ristig, D., Schuler, V., Meigel,
I., Lampert, C., Stein, C., Prezeau, L, Blahos, J., Pin, J.-P., Froestl, W., Kuhn, R., Heid, J., Kaupmann, K.,
Bettler B. (2001)
C-Terminal Interaction Is Essential for Surface Trafficking But Not for Heteromeric
Assembly of GABAB Receptors. J. Neurosci. 24: 1189-1202
8. Galvez, T., Duthey, B., Kniazeff, J., Blahos, J., Rovelli, G., Bettler, B., Prézeau, L., Pin, J.-P. (2001)
Allosteric Interactions Between GB1 and GB2 Subunits are Required for Optimal GABAB Receptor
Function. EMBO J. 20: 2152-2159
9. Havlickova, M., Prezeau, L., Duthey, B., Bettler, B., Pin, J.-P., Blahos, J. (2002)
The Intracellular Loops of
the GB2 Subunit are Crucial for G-protein Coupling of the Heteromeric GABAB Receptor. Mol. Pharmacol.
62: 343-350
|