Projects
Mechanisms of action of cytotoxic nucleoside / nucleotide analogs and mechanisms of resistance | |

|
In the long-term, this project aims to elucidate molecular basis of the antiproliferative activity of newly synthesized cytotoxic compounds. It starts by answering the questions whether and how the compounds are taken up by the cells, how they are metabolized and what are their active metabolites. We describe the apoptotic effects of such compounds as well as the effect on cell cycle distribution. We also follow the effect of the compounds on protein kinase- or caspase-mediated signaling, role of mitochondria, membrane 'death receptors' and interactions with telomeres / telomerase. Currently we focus on acyclic nucleoside phosphonate PMEG (a compound developed by Prof. Holý), whose double prodrug GS-9219 is under clinical trials as chemotherapeutics for malignant lymphoma. The knowledge of a compound's metabolism and mechanism of action allows us to identify cellular sites where the acquired resistance might originate from and give us an idea how to overcome this phenomenon. In resistant cells we study expression and function of membrane transporters of MRP and MDR class (e.g. P-glycoprotein), phosphorylation activity of key kinases and we also evaluate the sensitivity of resistant cells towards other commonly used cytostatics i.e. the ability of a compound to induce multidrug resistance. |
DNA methylation (epigenetics) | |

|
Epigenetics studies changes in gene expression caused by mechanisms that do not involve changes in DNA sequence. One of the major epigenetic mechanisms is represented by DNA methylation. Hypermethylation of 'CpG islands' located within the regulatory sequences of tumor suppressor genes frequently causes their silencing, which may lead to carcinogenesis. Restoring aberrant DNA methylation pattern with use of hypomethylating agents is expected to be more effective and less toxic method of cancer treatment compared to standard chemotherapy. Therefore, searching for new hypomethylating agents and reliable detecting methods is a top-priority task. |
Antiangiogenic effects of the compounds | |

|
Angiogenesis refers to new blood vessel formation based on existing vasculature. It is a complex, multi-step process that is influenced by a number of angiogenesis mediators (extracellular matrix components, growth factors, integrins, cytokines and enzymes) with inhibitory or stimulatory function. Angiogenesis dysregulation results in a number of pathological conditions such as rheumatoid arthritis or diabetic neuropathy. Last but not least, increased angiogenesis is necessary for the growth of solid tumors and their ability to form metastases. Traditionally we are dedicated to the identification of new thymidine phosphorylase (TP) inhibitors. This enzyme catalyzes the phosphorolysis of thymidine to thymine and 2'-deoxyribose-1-phosphate, which acts as chemoattractant. Angiogenic and antiapoptotic effects of TP (previously called PD-ECGF) are well known. Several 5-substituted 6-chlorouracil derivatives exerted a marked effect on human recombinant TP as well as on TP isolated from human placenta (Ki in submicromolar range, Nencka et al., 2007). Apart from TP inhibitors we also study the effect of selected compounds on other angiogenic factors such as VEGF. Currently we aim to set up new in vitro models for angiogenesis evaluation in cellular systems (endothelial cell migration and endothelial tube formation assays). |
Mitochondrial toxicity of nucleoside / nucleotide analogs | |
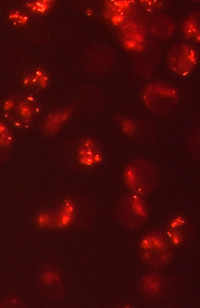
|
In this project we aim to investigate various mechanisms of mitochondrial toxicity of nucleoside / nucleotide analogues with antiviral or cytostatic activities. Numerous structurally related compounds have been previously implicated in mitochondrial dysfunction and this effect cannot be always explained solely on the basis of DNA polymerase γ inhibition. Events that trigger or accompany mitochondrial dysfunction involve not only damage to the mitochondrial DNA (mtDNA), but also loss of mitochondrial membrane potential (ΔΨm), cytochrome c dislocation, increased production of reactive oxygen species (ROS), etc. Here we will explore distinct characteristics of cytotoxicity presented by this type of compounds with a special focus on ROS-hyperproduction caused oxidative stress that contributes to accelerated telomere attrition and subsequent premature cellular senescence. Fluorescence-based analytical techniques will be employed for preliminary assessment of mitochondrial toxicity of newly synthesized compounds including measurement of reactive oxygen species (ROS) production and mitochondrial membrane potential (ΔΨm) in cell culture models and oxygen consumption by isolated mitochondria. In another part of the study we will focus on revealing relationship between different structural features of studied compounds and their potency to induce mitochondrial dysfunction, which is a common mechanism of drug-induced toxicity. Direct antioxidant or pro-oxidant properties of the compounds will also be evaluated. The acquired information is important in the rational design of new drugs with fewer side effects. |