|
|
|
Close Help | ||||||||||||||
|
|
|
Close Help | ||||||||||||||
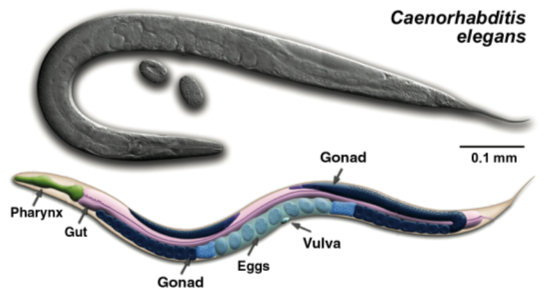
During differentiation, cells acquire new properties and become parts of organs and tissues. Work in model organisms such as the nematode Caenorhabditis elegans tells us what genes decide cell fate. The worm gonad is an interesting model to study cell fate decision, as its structure and function depends on asymmetric division of a single precursor cell. Each of its daughters acquires one of two possible fates: the distal cell will direct development of the gametes, while the proximal daughter induces the vulva. This asymmetry requires ß-catenin signaling; in its absence both daughters assume the proximal fate and consequently no gametes are formed. We have shown that specification of both developmental fates and thus making of the entire organ actually relies on interaction between the ß-catenin pathway and a nuclear receptor NHR-25. Loss of NHR-25 enables even totally sterile ß-catenin pathway mutants to develop distal cells and gametes. By binding ß-catenin, NHR-25 inhibits this signaling, whereas conversely ß-catenin blocks NHR-25 activity. Recent studies show that human and mouse NHR-25 homologues also interact with ß-catenin, thus modulating sexual development and cell cycle in mammals. Crosstalk between nuclear receptors and ß-catenin signaling therefore presents a broadly conserved mechanism of cell differentiation. The C. elegans model, however, has allowed us to demonstrate this molecular interaction with a single-cell resolution in vivo.
Asahina, M., Valenta, T., Silhankova, M., Korinek, V. and Jindra, M.:
Crosstalk between a nuclear receptor and ß-catenin signaling decides cell fates in the C. elegans somatic gonad.
Dev. Cell 11, 203-211 (2006)
Lukeš J., Vondrušková-Horáková E., Jirků M., Zíková A., Foldynová-Trantírková S., Verner Z., Paris Z., Hashimi H.
The protist Trypanosoma brucei is a causative agent of the African sleeping sickness, while closely related flagellates are responsible for a number of other serious diseases of man and animals. Present treatment of millions of people infected with these parasites is based on decades old therapeutics, against which resistance is popping up. The aim of our research is the identification of proteins essential for the parasite's survival that represent suitable pharmacological targets, since they have no homologues in the host cell. By means of RNA interference, we are studying proteins that participate in the processes unique to the trypanosome cell, such as RNA editing, RNA trans-splicing and respiration via an unusual respiratory chain. Using a number of phenotypic assays we have not only established which proteins are indispensable for the parasite, but also identified their function(s).
Lukeš J., Hashimi H. & Zíková A.:
Unexplained complexity of the mitochondrial genome and transcriptome in kinetoplastid flagellates.
Curr. Genet. 48: 277-299 (2005)
Horváth A., Horáková E., Dunajčíková P., Verner Z., Pravdová E., Šlapetová I., Cuninková L. & Lukeš J.:
Down-regulation of the nuclear-encoded subunits of the complexes III and IV disrupts their respective complexes but not complex I in procyclic Trypanosoma brucei.
Mol. Microbiol. 58: 116-130. (2005)
Foldynová-Trantírková S., Paris Z., Sturm N.R., Campbell D.A. & Lukeš J.:
The Trypanosoma brucei La protein is a candidate poly(U) shield protein that impacts spliced leader RNA maturation and tRNA intron removal.
Int. J. Parasitol. 35: 359-366 (2005)
Vondrušková E., van den Burg J., Zíková A., Ernst N.L., Stuart K., Benne R. & Lukeš J.:
RNA interference analyses suggest a transcript-specific regulatory role for MRP1 and MRP2 in RNA editing and other RNA processing in Trypanosoma brucei.
J. Biol. Chem. 280: 2429-2438 (2005)
Zíková A., Horáková E., Jirků M., Dunajčíková P. & Lukeš J.:
The effect of down-regulation of mitochondrial RNA-binding proteins MRP1 and MRP2 on respiratory complexes in procyclic Trypanosoma brucei.
Biochem. J. (submitted)
Moravec F., Wijová M., Horák A., Lukeš J., Bruňanská M.
Nematodes assigned so far to Dracunculoidea represent a large, diverse group of tissue parasites attacking members of all vertebrate classes including man. Despite their medical and veterinary medical importance, the present knowledge of their morphology, biology, distribution and phylogenetic relationships is by far incomplete. Detailed study of the morphology (including ultrastructure) and biology of many species from different hosts and geographical regions resulted in obtaining priority knowledge about these parasites, including the description of many new and redescription of inadequately known species (largely from fish), erection of several new genera, recognition of species diversity in so far little explored regions, and in obtaining new data on their life cycles and host-parasite relationships. Results of DNA studies in 17 representatives of different dracunculoid genera elucidated their phylogenetic interrelationships and, surprisingly, showed Anguillicolidae does not belong to dracunculoids. To date the first, comprehensive book monograph about dracunculoids and anguillicoloids has been worked out, where a new classification system of these parasites is presented; in addition to data on individual taxonomic groups and species, all-world identification keys are provided. This book is assumed to be a solid base for subsequent studies in this important parasite group.
Moravec F.:
Dracunculoid and anguillicoloid nematodes parasitic in vertebrates.
Academia, Praha (2006) (in press)
Frantová D., Bruňanská M. Fagerholm H.-P., Kihlström M.:
Ultrastructure of the body wall of female Philometra obturans (Nematoda: Dracunculoidea).
Parasitol. Res. 95: 327-332 (2005)
Moravec F., Nagasawa K., Miyakawa M.:
First record of ostracods as natural intermediate hosts of Anguillicola crassus, a pathogenic swimbladder parasite of eels Anguilla spp.
Dis. Aquat .Org. 66: 171-173 (2005)
Wijová M., Moravec F., Horák A., Modrý D., Lukeš J.:
Phylogenetic position of Dracunculus medinensis and some related nematodes inferred from 18S rRNA.
Parasitol. Res. 96: 133-135 (2005)
Moravec F.:
Some aspects of the taxonomy and biology of dracunculoid nematodes parasitic in fishes: a review.
Folia Parasitol. 51: 1-13 (2004)
Asahina M., Šilhánková M., Jindra M.
Integrity of the epidermis is critical for development and for survival of organisms that are constantly being challenged by adverse external conditions, both as free-living creatures and as parasites. Differentiation of epithelial tissues is a complex process governed by interacting signal transduction pathways. We use the nematode, Caenorhabditis elegans, to address the mechanisms of epidermal development in vivo.
We have found that a nuclear receptor NHR-25, whose homolog (SF-1) is well-studied in mammals for its role in sex differentiation and regulation of steroid hormone synthesis, exerts effects on epithelial differentiation and integrity. Deficiency of NHR-25 prevents epithelial stem cells to renew their adherens junction contacts, which are temporarily lost following asymmetric divisions of these cells. The lack of these cell-cell contacts results in abnormal fate specification of the stem cell daughters and in their excessive division. In addition, NHR-25 is necessary for proper cuticle synthesis and molting.
An apparently unrelated yet important Ras-like signaling pathway plays a similar role in epithelial integrity of the worm. We have shown that the C. elegans homologue of the mammalian guanine nucleotide exchange factors (GEFs), PXF-1, may function in polarized secretion, because loss of PXF-1 causes disorganized cuticle formation and defective molting. In both cases the C. elegans model reveals unprecedented roles for evolutionarily conserved signaling pathways with individual cell and tissue resolution.
Šilhánková M., Jindra M. and Asahina M.:
Nuclear receptor NHR-25 is required for cell-shape dynamics during epidermal differentiation in Caenorhabditis elegans.
J. Cell Sci. 118: 223-232 (2005)
Pellis-van Berkel W., Verheijen M.H., Cuppen E., Asahina M., de Rooij J., Jansen G., Plasterk R.H., Bos J.L., Zwartkruis F.J.:
Requirement of the Caenorhabditis elegans RapGEF pxf-1 and rap-1 for epithelial integrity.
Mol. Biol. Cell 16: 106-116 (2005)

 |
Biology Centre of the Academy of Sciences of the Czech Republic, v. v. i. |