|
APRIL 2011 -
J. Am. Chem. Soc. 2011, 133, 6130–6133.
Wieslaw J. Roth, Oleksiy V. Shvets, Mariya Shamzhy, Pavla Chlubná, Martin Kubů, Petr Nachtigall, and Jiří Čejka: Postsynthesis transformation of Three-Dimensional Framework into a Lamellar Zeolite with Modifiable Architecture (article here).
Abstract: Mild treatment of zeolite UTL results in degradation of its structure with preservation of the initially present dense layers connected by D4R “bridges”. The lamellar product obtained through this 3D to 2D zeolite conversion has been structurally modified similar to methodologies applied to layered zeolite precursors, which show the opposite 2D to 3D zeolite transformation.
Chem. Commun., 2011, 47, 5446–5448.
Antonín Trojánek, Jan Langmaier, Jakub Šebera, Stanislav Záliš, Jean-Michel Barbe, Hubert H. Giraultc and Zdeněk Samec: Fine tuning of the catalytic effect of a metal-free porphyrin on the homogeneous oxygen reduction (article here).
Abstract: The catalytic effect of tetraphenylporphyrin on the oxygen reduction with ferrocene in 1,2-dichloroethane can be finely tuned by varying the molar ratio of the acid to the catalyst present in the solution. The mechanism involves binding of molecular oxygen to the protonated free porphyrin base, in competition with ion pairing between the protonated base and the acid anion present.
ARCHIVE 2009-2011......
MARCH 2011 -
ACS Nano, 2011, 5 (3), pp 2231–2239.
Otakar Frank, Marcel Mohr, Janina Maultzsch, Christian Thomsen, Ibtsam Riaz, Rashid Jalil, Kostya S. Novoselov, Georgia Tsoukleri, John Parthenios, Konstantinos Papagelis, Ladislav Kavan, and Costas Galiotis: Raman 2D-Band Splitting in Graphene: Theory and Experiment (article here).
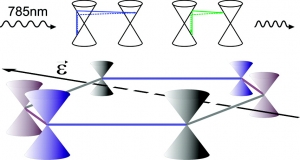
Abstract: We present a systematic experimental and theoretical study of the two-phonon (2D) Raman scattering in graphene under uniaxial tension. The external perturbation unveils that the 2D mode excited with 785 nm has a complex line-shape mainly due to the contribution of two distinct double resonance scattering processes (inner and outer) in the Raman signal. The splitting depends on the direction of the applied strain and the polarization of the incident light. The results give new insight into the nature of the 2D band and have significant implications for the use of graphene as reinforcement in composites since the 2D mode is crucial to assess how effectively graphene uptakes an applied stress or strain.
Coordination Chemistry Reviews 255, 7-8, 2011, 975–989.
Radka Baková, Majed Chergui, Chantal Daniel, Antonín Vlček Jr., Stanislav Záliš: Relativistic effects in spectroscopy and photophysics of heavy-metal complexes illustrated by spin–orbit calculations of [Re(imidazole)(CO)3(phen)]+ (article here).
Abstract: Spin–orbit coupling (SOC) is an essential factor in photophysics of heavy ransition metal complexes. By enabling efficient population of the lowest triplet state and its strong emission, it gives rise to a very interesting photophysical behavior and underlies photonic applications such as organic light emitting diodes (OLED) or luminescent imaging agents. SOC affects excited-state characters, relaxation dynamics, radiative and nonradiative decay pathways, as well as lifetimes and reactivity. We present a new photophysical model based on mixed-spin states, illustrated by relativistic spin–orbit TDDFT and MS-CASPT2 calculations of [Re(imidazole)(CO)3(1,10-phenanthroline)]+. An excited-state scheme is constructed from spin–orbit (SO) states characterized by their energies, double-group symmetries, parentages in terms of contributing spin-free singlets and triplets, and oscillator strengths of corresponding transitions from the ground state. Some of the predictions of the relativistic SO model on the number and nature of the optically populated and intermediate excited states are qualitatively different from the spin-free model. The relativistic excited-state model accounts well for electronic absorption and emission spectra of ReI carbonyl diimines, as well as their complex photophysical behavior. Then, we discuss the SO aspects of photophysics of heavy metal complexes from a broader perspective. Qualitative SO models as well as previous relativistic excited-state calculations are briefly reviewed together with experimental manifestations of SOC in polypyridine and cyclometallated complexes of second- and third row d6 metals. It is shown that the relativistic SO model can provide a comprehensive and unifying photophysical picture.
FEBRUARY 2011 -
Chemistry of Materials, 2011, 23 (2), 200-207 .
Petrykin V. , Macounova K. , Franc J. , Shlyakhtin O., Klementova M., Mukerjee S., Krtil P.: Zn-Doped RuO2 electrocatalyts for Selective Oxygen Evolution: Relationship between Local Structure and Electrocatalytic Behavior in Chloride Containing Media
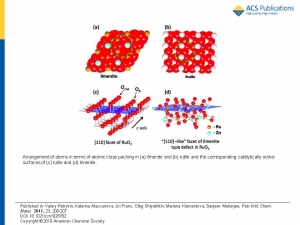
Abstract: Nanocrystalline electrocatalytically active materials of chemical composition
Ru1-xZnxO2 (0< x < 0.3) were synthesized by freeze-drying technique. The diffraction patterns of the prepared samples corresponded to single-phase rutile type oxides. Local structure of the Ru1-xZnxO2 based on refinement of Ru K and Zn K edge EXAFS functions shows clustering of the Zn ions in the blocks with ilmenite structure intergrowing with Ru-rich rutile blocks. Ru1- ZnxO2 oxides are selective catalysts for anodic oxygen evolution. The selectivity toward oxygen evolution in the presence of chlorides is affected by the actual Zn content and can be ascribed to structural hindrance of the formation of the surface peroxo group based active sites for chlorine evolution. The selectivity toward oxygen evolution in presence of chlorides is accompanied by the drop of the total activity, which gets more pronounced with
increasing Zn content (article here).
Phys. Chem. Chem. Phys., 2011, 13, 4365–4371.
Lubomír Pospıíšil, Magdaléna Hromadová, Nicolangelo Fanelli, Michal Valášek, Viliam Kolivoška and Miroslav Gál: Extended viologen as a source of electric oscillations.
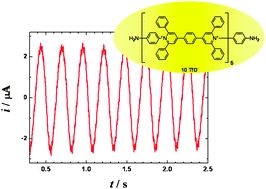
Abstract: A long organic molecule 1 with five bipyridinium functions separated by benzene rings (extended viologen) undergoes a reversible multi-step electron transfer. Here we show that this decacation accepts electrons at the heterogeneous interface with the occurrence of the periodically changing electric reduction currents. According to the applied bias voltage the observed current–time dependence changes from chaotic through periodic and irregular to sinusoidal and finally to monotonous. A careful choice of the controlling parameters yields the sustained periodic sinusoidal urrents lasting for a prolonged time. Oscillations stem from a mutual interplay of the heterogeneous supply of electrons and the homogeneous redox reactions (disproportionation) between the transient redox forms. In difference to many other electrochemical oscillating systems the described oscillations do not require any additional external impedance. The principle of these oscillatory currents may serve as a model of a truly ‘molecular oscillator’ (article here).
JANUARY 2011 -
Mass Spectrometry Reviews, 2011, Vol. 30 (2), pages 236–267.
Patrik Španěl, David Smith: Progress in SIFT-MS: Breath analysis and other applications.
Abstract: The development of selected ion flow tube mass spectrometry, SIFT-MS, is described from its inception as the modified very large SIFT instruments used to demonstrate the feasibility of SIFT-MS as an analytical technique, towards the smaller but bulky transportable instruments and finally to the current smallest Profile 3 instruments that have been located in various places, including hospitals and schools to obtain on-line breath analyses. The essential physics and engineering principles are discussed, which must be appreciated to design and construct a SIFT-MS instrument. The versatility and sensitivity of the Profile 3 instrument is illustrated by typical mass spectra obtained using the three precursor ions H3O+, NO+ and equation image, and the need to account for differential ionic diffusion and mass discrimination in the analytical algorithms is emphasized to obtain accurate trace gas analyses. The performance of the Profile 3 instrument is illustrated by the results of several pilot studies, including (i) on-line real time quantification of several breath metabolites for cohorts of healthy adults and children, which have provided representative concentration/population distributions, and the comparative analyses of breath exhaled via the mouth and nose that identify systemic and orally-generated compounds, (ii) the enhancement of breath metabolites by drug ingestion, (iii) the identification of HCN as a marker of Pseudomonas colonization of the airways and (iv) emission of volatile compounds from urine, especially ketone bodies, and from skin. Some very recent developments are discussed, including the quantification of carbon dioxide in breath and the combination of SIFT-MS with GC and ATD, and their significance. Finally, prospects for future SIFT-MS developments are alluded to (article here).
Anal. Chem., 2011, 83 (3), pp 1069–1077.
Martin Civiš, Svatopluk Civiš, Kristyna Sovová, Kseniya Dryahina, Patrik Šaněl, and
Martin Kyncl: Laser Ablation of FOX-7: Proposed Mechanism of Decomposition

Abstract: A novel high-energy explosive material, FOX-7 (1,1-diamino-2,2-dinitroethylene), was studied using a combination of laser-induced breakdown spectroscopy (LIBS) and selected ion flow tube mass spectrometry (SIFT-MS). The LIBS technique uses short laser pulses (an ArF excimer laser) as the energy source to convert small quantities of a sample into plasma and to induce the emission of its molecular fragments or atoms. SIFT-MS is a novel method for absolute quantification based on chemical ionization using three reagent ions, with the ability to determine concentrations of trace gases and vapors of volatile organic compounds in real time. SIFT-MS was used to study the release of NO, NO2, HCN, HONO, HCHO, CH3CH2OH, and C2H2 after laser ablation of the explosive compound FOX-7 in solid crystalline form. The radiation emitted after excitation was analyzed using a time-resolved UV−vis spectrometer with an ICCD detector. The electronic bands of CN (388 nm), OH (308.4 nm), and NO (237.1 nm) radicals and the atomic lines of C, N, and H were identified (article here).
DECEMBER 2010 -
J.Phys.Chem. B, 114, 2010, pp. pp 15773–15779.
J. Mosinger, K. Lang, J. Hostomský, J. Franc, J. Sýkora, M. Hof and P.Kubát:
Singlet Oxygen Imaging in Polymeric Nanofibers by Delayed Fluorescence.
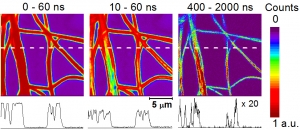
Abstract: Polymeric nanofiber materials loaded with photosensitizers exhibit significant antibacterial activity due to their generation of cytotoxic singlet oxygen O2(1Δg). A time-gated fluorescence imaging technique as used to monitor the photosensitized processes in polystyrene (PS) and gelatin (GE) nanofibers loaded with 0.1 wt % tetraphenylporphyrin (TPP) photosensitizer. The fluorescence decay of TPP at the periphery of the PS nanofibers was single exponential. Increased fluorescence quenching was observed in the domains with higher TPP loading, located in the center of the nanofibers, and added a shorter lifetime component to the kinetics. The domains exhibiting singlet oxygen activity within the nanofibers were visualized and analyzed by singlet oxygen-sensitized delayed fluorescence imaging (SODF). Whereas O2(1Δg) was produced in PS nanofibers, its production in GE nanofibers was limited. These results were confirmed by time-resolved phosphorescence measurements at 1270 nm (article here).
Our publication J. Chem. Phys.,2010, 133, 194106 is on "Top 20 Most Downloaded Articles" of the Journal of Chemical Physics (November 2010):
J. Chem. Phys., 2010, 133, 194106 (10 pages).
Libor Veis and Jiří Pittner: Quantum computing applied to calculations of molecular energies: CH2 benchmark
Abstract: Quantum computers are appealing for their ability to solve some tasks much faster than their classical counterparts. It was shown in [ Aspuru-Guzik et al., Science 309, 1704 (2005) ] that they, if available, would be able to perform the full configuration interaction (FCI) energy calculations with a polynomial scaling. This is in contrast to conventional computers where FCI scales exponentially. We have developed a code for simulation of quantum computers and implemented our version of the quantum FCI algorithm. We provide a detailed description of this algorithm and the results of the assessment of its performance on the four lowest lying electronic states of CH2 molecule. This molecule was chosen as a benchmark, since its two lowest lying 1A1 states exhibit a multireference character at the equilibrium geometry. It has been shown that with a suitably chosen initial state of the quantum register, one is able to achieve the probability amplification regime of the iterative phase estimation algorithm even in this case (article here).
NOVEMBER 2010 -
Journal of Catalysis 276, 2010, 327-334.
Jeong-Boon Koo, Nanzhe Jiang, Shunmugavel Saravanamurugan, Martina Bejblová, Zuzana Musilová, Jiří Čejka, Sang-Eon Park: Direct synthesis of carbon-templating mesoporous ZSM-5 using microwave heating.
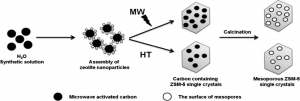
Abstract: Carbon-templated mesoporous ZSM-5 zeolites were synthesized directly avoiding a drying process. Carbon nanoparticles were simply mixed into synthesis precursor of ZSM-5 and hydrothermally treated by microwave irradiation. The amount of mesopores formed inside the ZSM-5 single crystals was controllable by adjusting the amount of carbon used. For comparison, mesoporous ZSM-5 zeolites have also been synthesized under hydrothermal conditions. The influence of microwave irradiation on mesoporous ZSM-5 materials was thoroughly investigated by using nitrogen adsorption/desorption studies and 27Al MAS NMR. The nature of acid sites both in the micropores (internal) and on the surface of mesopores (external) was investigated by in situ FTIR spectroscopy using pyridine (Py) and 2′,6′-di-tert-butylpyridine (DTBPy) as a probe molecules. Mesoporous ZSM-5 prepared by microwave synthesis showed higher catalytic activity in the bulky molecular reaction of ′,4′-dimethoxyacetophenone (2′,4′-DMAP) with 4-methoxybenzaldehyde as a model reaction in comparison with the results obtained over hydrothermally prepared ZSM-5. The further catalytic behavior has been studied in condensation reaction and cracking of substituted benzene (article here).
ACS Nano, 2010, 4 (10), pp 6055–6063.
Martin Kalbáč, Alfonso Reina-Cecco, Hootan Farhat, Jing Kong, Ladislav Kavan, and Mildred S. Dresselhaus: The Influence of Strong Electron and Hole Doping on the Raman Intensity of Chemical Vapor-Deposition Graphene
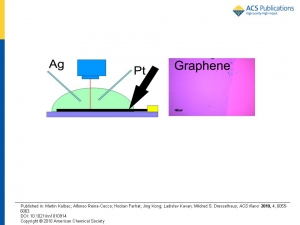
Abstract: Electrochemical charging has been applied to study the influence of doping on the intensity of the various Raman features observed in chemical vapor-deposition-grown graphene. Three different laser excitation energies have been used to probe the influence of the excitation energy on the behavior of both the G and G′ modes regarding their dependence on doping. The intensities of both the G and G′ modes exhibit a significant but different dependence on doping. While the intensity of the G′ band monotonically decreases with increasing magnitude of the electrode potential (positive or negative), for the G band a more complex behavior has been found. The striking feature is an increase of the Raman intensity of the G mode at a high value of the positive electrode potential. Furthermore, the observed increase of the Raman intensity of the G mode is found to be a function of laser excitation energy (article here).
OCTOBER 2010 -
Chem. Eur.J. 2010, 16, 11753 – 11759.
Martin Kalbáč, Ladislav Kavan, Sandeep Gorantla, Thomas Gemming, Lothar Dunsch: Sexithiophene Encapsulated in a Single-Walled Carbon Nanotube: An In Situ Raman Spectroelectrochemical Study of a Peapod Structure
Abstract: The interaction of single-walled carbon nanotubes (SWCNTs) and α-sexithiophene (6T) was studied by Raman spectroscopy and by in situ Raman spectroelectrochemistry. The encapsulation of 6T in SWCNT and its interaction causes a bleaching of its photoluminescence, and also small shifts of its Raman bands. The Raman features of the SWCNT with embedded 6T (6T-peapods) change in both intensity and frequency compared to those of pristine SWCNT, which is a consequence of a change of the resonant condition. Electrochemical doping demonstrated that the electrode potential applied to the SWCNT wall causes changes in the embedded 6T. The effects of electrochemical charging on the Raman features of pristine SWCNT and 6T SWCNT were compared. It is shown that the interaction of SWCNT with 6T also changes the electronic structure of SWCNT in its charged state. This change of electronic structure is demonstrated both for semiconducting and metallic tubes (article here). SWCNT were compared. It is shown that the interaction of SWCNT with 6T also changes the electronic structure of SWCNT in its charged state. This change of electronic structure is demonstrated both for semiconducting and metallic tubes (article here).
Organometallics, 2010, 29 (17), pp 3780–3789
R. Gyepes, V. Varga, M. Horáček, J. Kubišta, J. Pinkas and K. Mach: Influence of the Ti−O−C Angle on the Oxygen-to-Titanium π-Donation in [Cp2*Ti(III)OR] Complexes.
Abstract.
SEPTEMBER 2010 -
Journal of Physical Chemistry C, 2010, 114, 32, 13685-13694.
Brabec L ., Kočiřík M. :Silicalite-1 Crystals Etched with Hydrofluoric Acid Dissolved in Water or Acetone.
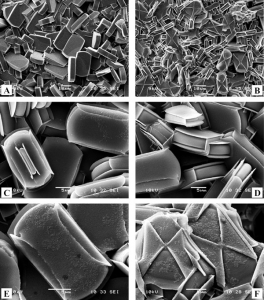
Abstract: Highly siliceous (Si/Al approximate to 350) MFI-type crystals of various sizes and morphologies were etched with HF dissolved either in water or in acetone. The highest concentration of HF used was 4 wt % in water and 5.5 wt % in acetone with etching time shorter than 1 h. After rapid etching, the lateral faces of coffin-shaped crystals exhibited differently resistant triangular areas. Slow etching lasting weeks or months was performed in 100 x diluted solutions and provided oriented rectangular pits corresponding to the point-group symmetry of MFI structure. Our coffin-shaped crystals were found to be twins of lateral faces as {l00} consisting of two pyramidal segments ingrown to the crystal bed. Inner resistant triangle skins were found in calcined crystals indicating preferential deposition of organic residues on the segment interface. Such skins also appeared in small flat monocrystals at the top of the calcined polycrystalline layer. They seem to be analogous to those found in coffin-shaped crystal twins. Etching of calcined crystals with HF-acetone solution led to dissolution of inner crystal bulk contrary to the impregnated outer shell. The etching patterns both on as-synthesized coffin-shaped crystals (surface triangles) and on calcined ones (inner triangles) resemble the well-known optical hourglass effect (article here).
Chemistry of Materials, 2010, 22, 13, 4045-4055.
Zukalova M., Prochazka J., Bastl Z., Duchoslav J., Rubacek L., Havlicek D., Kavan L.: Facile Conversion of Electrospun TiO2 into Titanium Nitride/Oxynitride Fibers
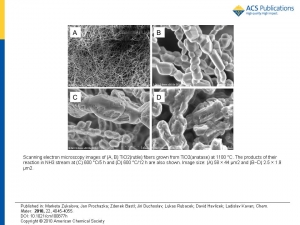
Abstract: Nanocrystalline fibrous TiO2 (anatase) was prepared by electrostatic spinning from ethanolic solution of Ti(IV) butoxide, acetylacetone, and poly(vinylpyrrolidone) employing the Nanospider industrial process. These titania fibers were smoothly converted into cubic titanium oxynitride, TiOxNy fibers (a = 4.1930 angstrom) during 4 h at 600 degrees C in ammonia atmosphere. The obtained material is convertible back into TiO2 fibers by heat treatment in air at 500 degrees C. The TiO2 fibers, which were reformed in this way, contain anatase as the main phase. Their follow-up reaction with NH3 at 600 degrees C/2 h leads to a less crystalline oxynitride material with a approximate to 4.173 angstrom, which is close to that of cubic TiO. Three subsequent cycles of this transformation were demonstrated. The described conversions are specific for electrospun anatase fibers only. At the same experimental conditions, other forms of nanocrystalline anatase do not react with ammonia yielding cubic phases. An almost perfectly stoichiometric titanium nitride, TiN (a = 4.2290 angstrom) containing only 0.2 wt % O, was prepared from TiOxNy fibers in NH3 at temperatures up to 1000 degrees C. This TiN material maintains the morphology of fibers and is composed of nanocrystals of a similar size as those of the precursor (article here).
AUGUST 2010 -
Recent Progress in Coupled Cluster Methods. Theory and Applications.
Čársky, Petr; Paldus, Josef; Pittner, Jiří (Eds.).
1st Edition., 2010, XXI, 650 p., Springer. ISBN: 978-90-481-2884-6

The coupled cluster method represents one of the most successful and often used approaches to a quantum-theoretical determination of atomic, molecular, and solid state electronic structure and properties. These methods are relevant to a broad spectrum of disciplines ranging from astrophysics to pharmacology...
This volume provides a useful source of reference for both researchers in chemistry, molecular physics and molecular biology and practitioners working in these fields. It is also recommendable to people using related software packages... (read more about this book).
Anal. Chem. 2010, 82, 5819-5829.
Alicia Olivares, Kseniya Dryahina, Jose Luis Navarro, Monica Flores, David Smith, and Patrik Španěl: Selected Ion Flow Tube-Mass Spectrometry for Absolute Quantification of Aroma Compounds in the Headspace of Dry Fermented Sausages (article here).

Figure: SIFT-MS full scan (FS) mass spectra obtained as the HS above a dry fermented sausage is introduced into the helium carrier gas using (a) H3O+ and (b) NO+ precursor ions. The individual characteristic product ions that appear, including their hydrates, are assigned to the analyte molecules indicated.
Abstract: The meat industry is interested in rapid control of the sensory quality of meat products. Selected ion flow tube mass spectrometry (SIFT-MS) has been applied to the rapid, real-time quantification of 31 volatile aroma compounds present in the headspace (HS) of dry fermented sausages. Three batches of fermented sausages with different fat contents (10, 20, and 30%) were monitored throughout the processing time. SIFT-MS revealed significant changes in the concentration of all the aroma compounds during the processing time. Moreover, among the various batches of the sausages, significant differences were revealed in their HS concentration of butanal, 2-pentenal, hexanal, 2-butanone, 2,3-butanedione, ethanol, ethyl formate, hexanoic acid, and dimethyl disulfide. In addition, highly volatile compounds were detected and
quantified using our real-time SIFT-MS technique that are apparently not generally seen by trace gas extraction and GC techniques.
JULY 2010 -
Angew. Chem. Int. Ed. 2010, 49, 4813-4815.
V. Petrykin, K. Macounova, O. A. Shlyakhtin, P. Krtil: Tailoring the Selectivity for Electrocatalytic Oxygen Evolution on Ruthenium Oxides by Zinc Substitution (article here).
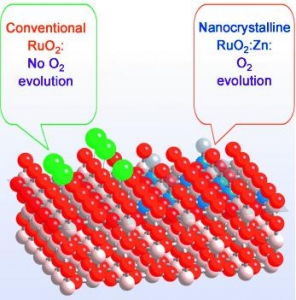
Abstract: Controlling gas emissions: Versatile control of the selectivity of an oxide electrocatalyst in the oxygen- and chlorine-evolution reactions was demonstrated by Zn substitution in RuO2 (see picture: O red, Cl green, Zn blue, Ru white). The incorporation of Zn into the rutile structure alters the cation sequence along the [001] direction and modifies the structure of the active sites for both gas-evolution processes.
Chem. Eur. J. 2010, 16, 7773 – 7780.
Dana Procházková, Martina Bejblová, Josef Vlk, Ajayan Vinu, Petr Štěpnička, Jiří Čejka: Selective Monoacylation of Ferrocene with Bulky Acylating Agents over Mesoporous Sieve AlKIT-5 (article here).
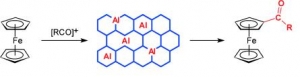
Abstract: Ferrocene acylation with bulky acylating agents (1-adamantoyl, benzoyl, 2-chlorobenzoyl, and cinnamoyl chlorides; and benzoic anhydride) catalyzed by AlKIT-5 mesoporous catalysts was investigated. AlKIT-5 catalysts with varying ratios of Si/Al were synthesized using tetraethoxysilane and aluminum isopropoxide as the structural building blocks and Pluronic F127 as a template under acidic conditions and were characterized in detail by X-ray powder diffraction, magic-angle spinning (MAS) NMR spectroscopy, sorption of nitrogen, energy-dispersive X-ray spectroscopy (EDS), SEM, TEM, and FTIR with pyridine as a probe molecule. The catalytic activity of the prepared AlKIT-5 catalysts in ferrocene acylation was shown to depend on the type of the acylating agent, thus likely reflecting the strength of interactions between the acyl source, the product, and the solid catalysts when the acylation reaction was carried out at 100 °C. In all reactions, the AlKIT-5 catalysts afforded exclusively the monoacylated products (100 % selectivity) most likely due to deactivation of the second cyclopentadiene ring by attachment of the first acyl group, steric reasons, and some competitive interactions of the monoacylferrocenes with the catalysts. The prepared AlKIT-5 catalysts could be regenerated without any significant loss of the ferrocene conversion.
JUNE 2010 -
Coordination Chemistry Reviews, 2010, 254, 1383–1396.
S. Záliš, R.F. Winter, W. Kaim: Quantum chemical interpretation of redox properties of ruthenium complexes with vinyl and TCNX type non-innocent ligands (article here).
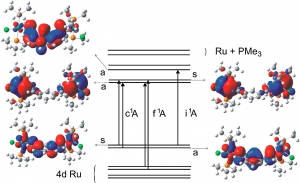
Abstract: This review provides an overview of density functional theory (DFT) calculations in a consequence with spectroelectrochemical measurements on mononuclear and symmetrically or unsymmetrically bridged di- and tetranuclear ruthenium complexes of vinyl and TCNX ligands. The DFT approach is used for the calculations of molecular structures, vibrational frequencies, electronic and electron paramagnetic resonance (EPR) spectral data. DFT calculations enable us to identity the primary redox site and the electron and spin-density distribution between the individual components for the individual redox congeners. The DFT technique reproduces the spectral properties of the presented complexes and their radical ions. The generally close correspondence between experimental and quantum chemical results demonstrate that modern DFT is a powerful tool to address issues like ligand non-innocence and electron and spin delocalization in systems containing both redox- ctive metal ions and redox-active ligands.
Journal of Catalysis, 2010, 272 (2), 262-274.
Š. Sklenák, P. C. Andrikopoulos, B. Boekfa, B. Jansang, J. Nováková, L. Benco, T. Bucko, J. Hafner, J. Dědeček, Z. Sobalík: N2O decomposition over Fe-zeolites: Structure of the active sites and the origin of the distinct reactivity of Fe-ferrierite, Fe-ZSM-5, and Fe-beta. A combined periodic DFT and multispectral study (article here).
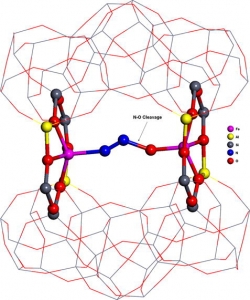
Abstract: The N2O decomposition over Fe-ferrierite, Fe-beta, and Fe-ZSM-5 has been recently studied [K. Jisa, J. Novakova, M. Schwarze, A. Vondrova, S. Sklenak, Z. Sobalik, J. Catal. 262 (2009) 27] and a superior activity of Fe-ferrierite with respect to Fe-beta and Fe-ZSM-5 has been shown. In this study, we investigated (1) plausible active sites for the N2O decomposition over Fe-ferrierite and (2) the origin of the distinct reactivity of Fe-ferrierite, Fe-ZSM-5 and Fe-beta employing a combined theoretical (periodic DFT) and experimental (UV–vis–NIR spectroscopy, IR spectroscopy, 29Si MAS NMR spectroscopy and catalytic batch experiments) approach. We evidenced that two Fe(II) cations accommodated in two adjacent six-membered rings in the eight-membered ring channel (β sites) of Fe-ferrierite (the calculated Fe–Fe distance is 7.4 Å) form the active site responsible for the superior activity of this catalyst in the N2O decomposition in the absence of NO. Similar structures can be formed in Fe-beta. However, the probability of their formation is very low. For Fe-ZSM-5, the geometrical arrangement of the cationic positions is far from that in Fe-ferrierite and it is not suitable for the N2O decomposition. Therefore, the predicted order of the activity of the Fe(II) exchanged zeolites agrees with our experimental findings and it is: Fe-ferrierite much greater-than Fe-beta > Fe-ZSM-5. We further showed that the accommodation of divalent cations in rings forming cationic sites can lead to significant rearrangements of the local structures of the zeolite framework, and therefore, the precise structure of sites binding a divalent cation cannot be derived from results of X-ray diffraction experiments, but can be inferred from theoretical calculations.
MAY 2010 -
BBA - Biomembranes, Vol.1798, 7, 2010, 1377-1391.
Radek Macháň, Martin Hof: Lipid diffusion in planar membranes investigated by fluorescence correlation spectroscopy (artile here).
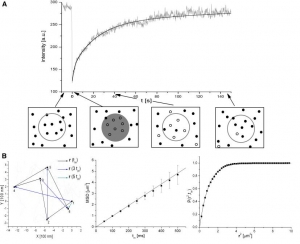
Abstract: Investigation of lipid lateral mobility in biological membranes and their artificial models provides information on membrane dynamics and structure; methods based on optical microscopy are very convenient for such investigations. We focus on fluorescence correlation spectroscopy (FCS), explain its principles and review its state of the art versions such as 2-focus, Z-scan or scanning FCS, which overcome most artefacts of standard FCS (especially those resulting from the need for an external calibration) making it a reliable and versatile method. FCS is also compared to single particle tracking and fluorescence photobleaching recovery and the applicability and the limitations of the methods are briefly reviewed. We discuss several key questions of lateral mobility investigation in planar lipid membranes, namely the influence which membrane and aqueous phase composition (ionic strength and sugar content), choice of a fluorescent tracer molecule, frictional coupling between the two membrane leaflets and between membrane and solid support
(in the case of supported membranes) or presence of membrane inhomogeneities has on the lateral mobility of lipids. The recent FCS studies addressing those questions are reviewed and possible explanations of eventual discrepancies are mentioned.
Chem. Mater., 2010, 22 (11), pp 3482–3495.
Oleksiy V. Shvets, Natalia Kasian, Arnošt Zukal, Jiří Pinkas, and Jiří Čejka: The Role of Template Structure and Synergism between Inorganic and Organic Structure Directing Agents in the Synthesis of UTL Zeolite (article here).
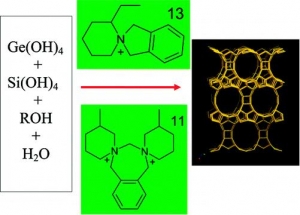
Abstract: The extra-large-pore germanosilicates with UTL topology have been synthesized using a large variety of spiroazocompounds as structure-directing agents. Synthesis conditions were optimized and zeolites with a high crystallinity degree were obtained with 13 different organic structure-directing agents. The influence of the composition of the reaction mixture and template nature (structure, hydrophilicity/hydro-phobicity balance, rigidity, pKa) on the phase selectivity, crystallinity degree, and adsorption properties of zeolites with UTL structure was investigated. Selection criteria of organic molecules as potential structure-directing agents (SDAs) in the synthesis of large-pore and extra-large-pore zeolites from silicate and germanosilicate media are proposed. The optimum synthesis time was determined to be 4−9 days for different SDA and (Si + Ge)/SDA molar ratios. Clear synergism between the optimum structure of organic template and the presence of critical amount of inorganic component (GeO2) was evidenced. The UTL zeolite crystallizes as tiny sheets 10 μm thick. The effect of the organic template on the size and shape of the crystals was found. The micropore volume of the best crystals is 0.22−0.24 cm3/g, with a micropore diameter of 1.05 nm, based on density functional theory (DFT), and Saito−Foley analyses of adsorption isotherms.
APRIL 2010 -
Phys. Chem. Chem. Phys., 2010, 12, 5240-5247.
Arnošt Zukal, Jana Mayerová and Jiří Čejka: Alkali metal cation doped Al-SBA-15 for carbon dioxide adsorption (article here).
Abstract: Mesoporous aluminosilicate adsorbents for carbon dioxide were prepared by the grafting of aluminium into SBA-15 silica using an aqueous solution of aluminium chlorohydrate. As the ion exchange sites are primarily associated with the presence of tetrahedrally coordinated aluminium, extra-framework aluminium on the SBA-15 surface was inserted into the silica matrix by a treatment with an aqueous solution of NH4OH. Synthesized mesoporous aluminosilicate preserving all the characteristic features of a mesoporous molecular sieve was finally modified by the alkali metal cation exchange. To examine carbon dioxide adsorption on prepared materials, adsorption isotherms in the temperature range from 0 °C to 60 °C were measured. Based on the known temperature dependence of adsorption isotherms, isosteric adsorption heats giving information on the surface energetics of CO2 adsorption were calculated and discussed. The comparison of carbon dioxide isotherms obtained on aluminosilicate SBA-15, aluminosilicate SBA-15 containing cations Na+ and K+ and activated alumina F-200 reveals that the doping with sodium or potassium cations dramatically enhances adsorption in the region of equilibrium pressures lower than 10 kPa. Therefore, synthesized aluminosilicate adsorbents doped with Na+ or K+ cations are suitable for carbon dioxide separation from dilute gas mixtures.
Zeolites and Catalysis: Synthesis, Reactions and Applications
Jiří Čejka (Editor), Avelino Corma (Editor), Stacey Zones (Editor). Wiley, ISBN:
978-3-527-32514-6, 918 pages, April 2010.

This indispensable two-volume handbook covers everything on this hot research field.
The first part deals with the synthesis, modification, characterization and application of catalytic active zeolites, while the second focuses on such reaction types as cracking, hydrocracking, isomerization, reforming and other industrially important topics. Edited by a highly experienced and internationally renowned team with chapters written by the "Who's Who" of zeolite research (more info about book...).
MARCH 2010 -
Angew. Chem. Int. Ed. 2010, 49, 2937 –2940.
Martin Lamač, Anke Spannenberg, Haijun Jiao, Sven Hansen, Wolfgang Baumann, Perdita Arndt, Uwe Rosenthal: Formation of a 1-Zircona-2,5-disilacyclopent-3-yne: Coordination of 1,4-Disilabutatriene to Zirconocene? (article here)
Abstract: Alkyne under stress: A novel metallacycle containing one Zr atom, two Si
atoms, and a CC bond has been prepared and its structure elucidated. According to X-ray data, spectral properties, and DFT calculations, the bonding situation in this compound is characterized as a 1-metalla-2,5-disilacyclopent-3-yne with a weak metal-triple-bond interaction.
Phys. Chem. Chem. Phys., 2010, 12 (13), 3145-3155.
Votava O., Mašát M., Pracna P., Kassi S., Campargue A.: Accurate determination of low state rotational quantum numbers (J < 4) from planar-jet and liquid nitrogen cell absorption spectra of methane near 1.4 micron (article here).
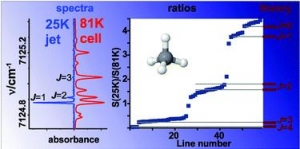
Abstract: An improved procedure for accurate determination of empirical lower state rotational quantum numbers from molecular absorption spectra is demonstrated for methane. We combine the high resolution absorption spectra in the 7070-7300 cm(-1) frequency range obtained in liquid nitrogen cooled cryogenic cell (T = 81 K) and in supersonic planar jet expansion (T-R = 25 K). Empirical lower state energies of 59 transitions are determined from the ratio of the absolute absorption line strengths at 25 and 81 K. The procedure relies on the realistic description of rotational state populations in the supersonic jet expansion where non-equilibrium nuclear spin isomer distributions are generated due to the rapid cooling. The accuracy of the experimental determination of the lower state energies with J <= 3 is found to considerably improve the results of the same approach applied to spectra at 296 and 81 K. The 59 transitions with determined lower J values provide a good starting point for the theoretical interpretation of the highly congested icosad region of methane. In particular, the centres of nine vibrational bands are estimated from the transitions with J = 0 lower state rotational quantum number.
FEBRUARY 2010 -
J.Am.Chem. Soc., 2010,132(8), 2655–2662.
Bin Su, Imren Hatay, Antonín Trojánek, Zdeněk Samec, Tony Khoury, Claude P. Gros, Jean-Michel Barbe, Antoine Daina, Pierre-Alain Carrupt and Hubert H. Girault: Molecular Electrocatalysis for Oxygen Reduction by Cobalt Porphyrins Adsorbed at Liquid/Liquid Interfaces (article here).
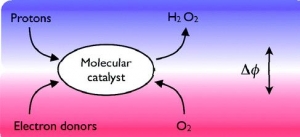
Abstract: Molecular electrocatalysis for oxygen reduction at a polarized water/1,2- dichloroethane (DCE) interface was studied, involving aqueous protons, ferrocene (Fc) in DCE and amphiphilic cobalt porphyrin catalysts adsorbed at the interface. The catalyst, (2,8,13,17-tetraethyl-3,7,12,18-tetramethyl-5-p-amino-phenylporphyrin) cobalt(II) (CoAP), functions like conventional cobalt porphyrins, activating O2 via coordination by the formation of a superoxide structure. Furthermore, due to the hydrophilic nature of the aminophenyl group, CoAP has a strong affinity for the water/DCE interface as evidenced by lipophilicity mapping calculations and surface tension measurements, facilitating the protonation of the CoAP−O2 complex and its reduction by ferrocene. The reaction is electrocatalytic as its rate depends on the applied Galvani potential difference between the two phases.
JANUARY 2010 -
Phys. Chem. Chem. Phys., 20100, 12, 1550-1556.
Lubomír Pospíšil, Filip Teplý, Miroslav Gál, Louis Adriaenssens, Michal Horáček and Lukáš Severa: Helquats, helical extended diquats, as fast electron transfer systems (article here).
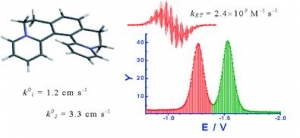
Abstract:
Helicene-viologen structural hybrids, like [5]helquat, 6,7,10,11-tetrahydrodipyrido[2,1-a:1´,2´-k][2,9]phenanthrolinediium, and its four methylated derivatives, are characterized by electrochemical admittance and EPR spectroscopy. All compounds are reversibly reduced in two one-electron steps. Formal redox potentials correlate with the calculated LUMO energies. The electron transfer is coupled with a weak adsorption of the reactants. The analysis of the frequency dependence of the electrode admittance is used for the separation of Faradaic and double layer contributions and finally to the estimation of heterogeneous rate constants. Heterogeneous rate constants determined this way are in the range 0.1 to 3 cm s-1. In all cases the second electron transfer is faster than the first redox step by a factor of three. The Frumkin correction for the acceleration by the double layer potential further amplifies this difference. The heterogeneous rate constants of derivatives correlate with the solvent reorganization energy estimated from the Marcus model. EPR spectra confirm the radical cation formation. The radical of [5]helquat participates in an extremely fast self-exchange process with the parent dication characterized by the self-exchange rate constant kET = (2.4 ± 0.5) × 109 M-1 s-1.
ACS Nano, 2010, 4 (1), pp. 459-469.
Martin Kalbáč, Alexander A. Green, Mark C. Hersam, and Ladislav Kavan: Tuning of Sorted Double-Walled Carbon Nanotubes by Electrochemical Charging (article here).

Abstract: Double-walled carbon nanotubes sorted by density gradient ltracentrifugation were examined by Raman spectroscopy and by in situ Raman pectroelectrochemistry. The sorted samples had a narrow distribution of diameters of oth inner and outer tubes, which enabled a comparison of the behavior of inner metallic tubes and inner semiconducting nanotubes as a function of the applied electrochemical potential. The metallic inner tubes were efficiently doped even though they were protected from electrolyte ions by the outer wall, whereas the doping of emiconducting inner tubes was observed only at high magnitudes of the electrode otential. These results indicate that the doping response of inner tubes is redominantly controlled by inner tube electronic properties. On the other hand, the effect of electronic structure of the outer tube on the behavior of inner tube is weak. Furthermore, the fficiency of the charge transfer from outer to inner wall depends on the doping level. A low doping level corresponds to a high efficiency of the charge-transfer, while a high doping level shows low charge-transfer efficiency.
DECEMBER 2009 -
CARBON, 2009, 48 (1), 153-162.
Pospisil L, Hromadova M, Gal M, et al.: Redox potentials and binding enhancement of fullerene and fullerene-cyclodextrin systems in water and dimethylsulfoxide (article here).
Abstract: The formal redox potentials of electron transfer reactions of fullerene, methanofullerene, ullerene–cyclodextrin complex and methanofullerene conjugates with cyclodextrins in aqueous and imethylsulfoxide solutions are reported. These new compounds are surface active and retain the redox activity of C60 even in aqueous medium. Compounds have been characterized by an electrochemical dmittance technique, which offers an advantage of separating faradaic and capacitive properties. Observed difference of formal redox potentials of the free fullerene forms and their cyclodextrin- ontaining compounds were used to determine the binding enhancement. Results are interpreted in terms of inter-molecular host–guest interactions of C60-cyclodextrin conjugates.
NOVEMBER 2009 -
Biophysical Journal, 2009, 97 ( 9), 2623-2629.
Jana Humpolíčková, Aleš Benda and Jörg Enderlein: Optical Saturation as a Versatile Tool to Enhance Resolution in Confocal Microscopy (article here).
Abstract:
One of the most actively developing areas in fluorescence microscopy is the achievement of spatial resolution below Abbe's diffraction limit, which restricts the resolution to several hundreds of nanometers. Most of the approaches in use at this time require a complex optical setup, a difficult mathematical treatment, or usage of dyes with special photophysical properties. In this work, we present a new, to our knowledge, approach in confocal microscopy that enhances the resolution moderately but is both technically and computationally simple. As it is based on the saturation of the transition from the ground state to the first excited state, it is universally applicable with respect to the dye used. The idea of the method presented is based on a principle similar to that underlying saturation excitation microscopy, but instead of applying harmonically modulated excitation light, the fluorophores are excited by picosecond laser pulses at different intensities, resulting in different levels of saturation. We show that the method can be easily combined with the concept of triplet relaxation, which by tuning the dark periods between pulses helps to suppress the formation of a photolabile triplet state and effectively reduces photobleaching. We demonstrate our approach imaging GFP-labeled protein patches within the plasma membrane of yeast cells.
Chem. Soc. Rev., 2009, 38, 3373-3382.
W. Kaim and J. Fiedler: Spectroelectrochemistry: the best of two worlds (article here).
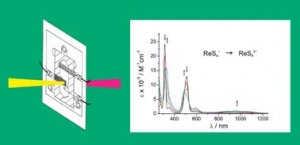
Abstract:
The combination of reaction-oriented electrochemistry with species-focussed spectroscopy in spectroelectrochemistry (SEC) allows for a more complete analysis of single and multiple electron-transfer processes and redox reactions in general. Practical considerations and guidelines are provided in this tutorial review, and selected examples involving UV-VIS-NIR and IR absorption spectroscopy as well as electron paramagnetic resonance (EPR) are presented to illustrate the potential and the applicability of this technique.
OCTOBER 2009 -
ACS Nano, 2009, 3 (8), pp. 2320-2328.
Martin Kalbac, Hootan Farhat, Ladislav Kavan, Jing Kong, Ken-ichi Sasaki, Riichiro
Saito and Mildred S. Dresselhaus: Electrochemical Charging of Individual Single-Walled Carbon Nanotubes (article here).
Abstract:
The influence of the electrode potential on the electronic structure of individual single-walled carbon nanotubes is studied using Raman spectroscopy. By analyzing the radial breathing mode intensity versus electrode potential profiles in the Raman spectra at many different laser excitation energies, we show that the charging of individual carbon nanotubes causes a broadening of the resonant Raman profiles (resonance window). This effect is observed for both a semiconducting and a metallic tube. The broadening of the resonance Raman profiles already begins at potentials where the first electronic states of a particular tube are filled or depleted. The important consequence of this effect is a striking difference between the Raman intensity versus potential profiles of metallic and semiconducting tubes. While for a metallic tube the intensity of the Raman signal is attenuated at potentials which deviate slightly from 0 V, for a semiconducting tube, the Raman intensity is significantly attenuated only after the electrode potential reaches the first van Hove singularity. Furthermore, for the metallic tube, a strong asymmetry is found in the bleaching of the Raman signal with respect to positive and negative potentials, which results from the different energy bandwidth for the π* band and the π band.
J. Am. Chem. Soc. , 2009, 131 (33), pp 11788–11800.
Ana Mara Blanco-Rodrguez, Michael Busby, Kate Ronayne, Michael Towrie, Cristian Grdinaru, Jawahar Sudhamsu, Jan Sýkora, Martin Hof, Stanislav Záliš, Angel J. Di Bilio, Brian R. Crane, Harry B. Gray and Antonín Vlček, Jr.: Relaxation Dynamics of Pseudomonas aeruginosa ReI(CO)3(α-diimine)(HisX)+ (X = 83, 107, 109, 124, 126)CuII Azurins (article here).
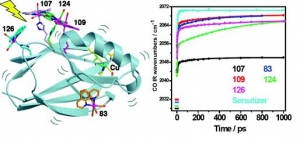
Abstract: Photoinduced relaxation processes of five structurally characterized Pseudomonas aeruginosa ReI(CO)3(α-diimine)(HisX) (X = 83, 107, 109, 124, 126)CuII azurins have been investigated by time-resolved (ps−ns) IR spectroscopy and emission spectroscopy. Crystal structures reveal the presence of Re-azurin dimers and trimers that in two cases (X = 107, 124) involve van der Waals interactions between interdigitated diimine aromatic rings. Time-dependent emission anisotropy measurements confirm that the proteins aggregate in mM solutions (D2O, KPi buffer, pD = 7.1). Excited-state DFT calculations show that extensive charge redistribution in the ReI(CO)3 → diimine 3MLCT state occurs: excitation of this 3MLCT state triggers several relaxation processes in Re-azurins whose kinetics strongly depend on the location of the metallolabel on the protein surface. Relaxation is manifested by dynamic blue shifts of excited-state ν(CO) IR bands that occur with triexponential kinetics: intramolecular vibrational redistribution together with vibrational and solvent relaxation give rise to subps, 2, and 8−20 ps components, while the 102 ps kinetics are attributed to displacement (reorientation) of the ReI(CO)3(phen)(im) unit relative to the peptide chain, which optimizes Coulombic interactions of the ReI excited-state electron density with solvated peptide groups. Evidence also suggests that additional segmental movements of Re-bearing β-strands occur without perturbing the reaction field or interactions with the peptide. Our work demonstrates that time-resolved IR spectroscopy and emission anisotropy of ReI carbonyl−diimine complexes are powerful probes of molecular dynamics at or around the surfaces of proteins and protein−protein interfacial regions.
SEPTEMBER 2009 -
Analytical Chemistry, 2009, 81, 6382-6389.
Langmaier J., Samec Z.: Voltammetry of Ion Transfer across a Polarized Room-Temperature Ionic Liquid Membrane Facilitated by Valinomycin: Theoretical Aspects and Application (article here).
Abstract: Cyclic voltammetry is used to investigate the transfer of alkali-metal cations, protons, and ammonium ions facilitated by the complex formation with valinomycin at the interface between an aqueous electrolyte solution and a room-temperature ionic liquid (RTIL) membrane. The membrane is made of a thin (112 μm) microporous filter impregnated with an RTIL that is composed of tridodecylmethylammonium cations and tetrakis[3,5-bis(trifluoromethyl)phenyl]borate anions. An extension of the existing theory of voltammetry of ion transfer across polarized liquid membranes makes it possible to evaluate the standard ion-transfer potentials for the hydrophilic cations studied, as well as the stability constants (Ki) of their 1:1 complexes with valinomycin, as log Ki = 9.0 (H+), 11.1 (Li+), 12.8 (Na+), 17.2 (K+), 15.7 (Rb+), 15.1 (Cs+), and 14.7 (NH4+). These data point to the remarkably enhanced stability of the valinomycin complexes within RTIL, and to the enhanced selectivity of valinomycin for K+ over all other univalent ions studied, compared to the conventional K+ ion-selective liquid-membrane electrodes. Selective complex formation allows one to resolve voltammetric responses of K+ and Na+ in the presence of an excess of Mg2+ or Ca2+, which is demonstrated by determination of K+ and Na+ in the table and tap water samples.
Analytical Chemistry, 2009, 81, 6327-6333.
Mikysek T., Švancara I., Klacher K., Bartoš M., Vytřas K. Ludvík J.: New Approaches to the Characterization of Carbon Paste Electrodes Using the Ohmic Resistance Effect and Qualitative Carbon Paste Indexes (article here).
Abstract: In this article, some new approaches to characterize the carbon paste mixtures and the respective carbon paste electrodes (CPEs) are presented, discussed, and critically evaluated. Particular attention has been paid to the changes of the ohmic resistance, relative to the dependence on composition of the CPE, the materials used, the time, and the position of storage. Four types of carbon pastes were examined, and for the interpretation of experimental data, a new simple model of “close-packing of spheres” has been applied. This model resembles the percolation theory for solid matter. In our case, however, it is possible to explain not only the “bent” or “broken” shape of the dependence of the electrode resistance upon the binder:carbon ratio and the corresponding electrochemical current response, but also differences caused by various material used and three various effects observed during the electrode aging. Furthermore, the report presents the significance of practical utilization of the recently introduced carbon paste index (denoted as χCPE), which is a qualitative hitherto unused factor based on the evaluation of cyclic voltammograms for standard redox systems (e.g., [Fe(CN)6]3−/4−) and specifying the electrochemical properties of a CPE. Some problems connected with homogeneity and stability of carbon pastes, their handling, storage, or eventual aging effects are also discussed.
AUGUST 2009 -
Biophysical Journal, 2009, 97 (3), L1-L3.
Štefl M., Kułakowska A., Hof M.: Simultaneous Characterization of Lateral Lipid and Prothrombin Diffusion Coefficients by Z-Scan Fluorescence Correlation Spectroscopy (article here).
Abstract: A new (to our knowledge) robust approach for the determination of lateral diffusion coefficients of weakly bound proteins is applied for the phosphatidylserine specific membrane interaction of bovine prothrombin. It is shown that z-scan fluorescence correlation spectroscopy in combination with pulsed interleaved dual excitation allows simultaneous monitoring of the lateral diffusion of labeled protein and phospholipids. Moreover, from the dependencies of the particle numbers on the axial sample positions at different protein concentrations phosphatidylserine-dependent equilibrium dissociation constants are derived confirming literature values. Increasing the amount of membrane-bound prothrombin retards the lateral protein and lipid diffusion, indicating coupling of both processes. The lateral diffusion coefficients of labeled lipids are considerably larger than the simultaneously determined lateral diffusion coefficients of prothrombin, which contradicts findings reported for the isolated N-terminus of prothrombin.
Journal of Catalysis, 2009, 266, 79-91.
Zilkova N., Bejblova M., Gil B., Zones S.I., Burton A. W., Chen C. Y., Musilova-Pavlackova Z., Kosova G., Cejka J.: The role of the zeolite channel architecture and acidity on the activity and selectivity in aromatic transformations: The effect of zeolite cages in SSZ-35 zeolite (article here).
Abstract: A series of zeolites differing in the channel architecture and acidity was investigated in toluene disproportionation, together with toluene and p-xylene alkylation with isopropyl alcohol. Zeolites with one- to three-dimensional 10-ring and 12-ring channels with and without cages, and those having 12–12–10 and 12–10–10-ring channel systems were studied. It was shown that general relationship of increasing zeolite activity with increasing pore diameter and pore connectivity is not valid as the size of some 12-ring channels (Beta, MCM-68) is comparable with 10-ring channels (ZSM-5, SSZ-35). In addition, the presence of cages in the structure of SSZ-35 and MCM-58 attributes the unusual catalytic behavior of these zeolites. SSZ-35 and MCM-58 zeolites behave in both toluene reactions such as three-dimensional large-pore zeolites. Subtle differences between zeolites of similar pore sizes and dimensionality can be usually explained based on the differences in the acidity of the individual zeolites. In p-xylene alkylation SSZ-35 exhibited high conversion with the highest selectivity to 1-isopropyl-2,5-dimethyl benzene and a low rate of deactivation. The presence of 18-ring cages in the channels of 10-ring zeolite SSZ-35 (STF) gives rise to an unusual catalytic behavior of this zeolite by comparison to other 10-ring zeolites. SSZ-35, possessing channels of 0.54 × 0.57 nm in diameter, exhibits catalytic activity in transformation of aromatic hydrocarbons that is similar to large-pore
zeolites. 18-Ring cages enable the formation of relatively bulkier transition states while the diffusion of the product molecules out of the 10-ring channel system is not slowed down due to 10-ring windows. In addition, the channel system of SSZ-35 prevents the formation of coke precursors.
JULY 2009 -
Journal of Catalysis, 2009, 262 (1), 27-34.
Jisa K., Novakova J., Schwarze M., Vondrova A., Sklenak S., Sobalik Z.: Role of the Fe-zeolite structure and iron state in the N2O decomposition: Comparison of Fe-FER, Fe-BEA, and Fe-MFI catalysts (article here).
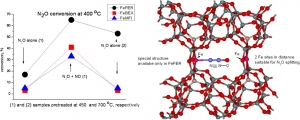
Abstract: The decomposition of nitrous oxide was compared over Fe-FER, Fe-MFI, and Fe-BEA with well established iron distribution in cationic positions and low amounts of less well-established oxide species. It was evidenced that, despite a comparable content of Fe(II) in the cationic positions, the catalytic activity of re-FER greatly exceeds that of Fe-BEA and Fe-MFI. While about one half of the iron sites in Fe-FER (Fe/Al < 0.15) participate in the decomposition of nitrous oxide after activation at 450 degrees C, the number of active sites in Fe-BEA or Fe-MFI was much lower, and, accordingly, without acceleration of the reaction by the addition of NO, these samples exhibit much lower catalytic activity than Fe-FER. This could be likely correlated with the concentration of Fe(II) in positions with a specific spatial iron arrangement at optimal Fe center dot center dot center dot Fe distances. For that role we propose a local structure with two adjacent beta sites, where the Fe center dot center dot center dot Fe distance would be 7 to 7.5 angstrom, i.e. comparable to the length of the N2O molecule, and provide potential for cooperation of the two iron cations on the N2O Molecule splitting. Such arrangement is absent in both the Fe-BEA and re-MFI structures.
JUNE 2009 -
Chemistry of Materials, 2009, 21 (8), 1457-1464.
Prochazka J., Kavan L., Zukalova M., Frank O., Kalbac M., Zukal A., Klementova M., Carbone D.,
Graetzel M.: Novel Synthesis of the TiO2(B) Multilayer Templated Films.
Abstract: TiO2(B) mesoporous thin films were grown in two steps on the F-doped SnO2 conductive glass substrates. In the first step, a small amount of H3PO4, corresponding to 0.15-0.375 wt % P on TiO2 basis, was introduced into concentrated HCl which was subsequently used for hydrolysis of titanium ethoxide. The hydrolyzed colloidal TiO2 Suspension was further mixed with a 1-butanol solution of the amphiphilic triblock copolymer Pluronic P123. The obtained precursor mixture was used for dip coating of FTO substrates. To achieve over 1 mu m thick films, dip coating (followed by a thermal treatment at 350 degrees C/2 h) was repeated several times to produce multilayer films. The films consisted of amorphous TiO2 with small amounts of anatase and TiO2(B). The amorphous part was converted into the TiO2(B) in a simple firing step at 500-550 degrees C. The formation of TiO2(B) phase was accompanied by a significant increase of the film thickness. The films demonstrated unique behavior during the electrochemical lithium insertion that would qualify them for fast battery or electrochromic smart window applications. The efficiency of multiphase TiO2 films in dye sensitized solar cells depends on the composition of individual films: it increases in the series: anatase/ amorphous TiO2 < anatase/TiO2(B) < a
|
 Struktura
Struktura  Oddělení
Oddělení  Lidé
Lidé  Ocenění
Ocenění  Výroční zprávy
Výroční zprávy  Hodnocení ústavu
Hodnocení ústavu  Knihovna
Knihovna  Média
Média  Popularizace
Popularizace  Výběrová řízení
Výběrová řízení