Projects
Duplex cyclodextrinsThe aim of the project is the preparation of cyclodextrin dimers connected in a head-to-head fashion by short multiple linkers. The disulfide bond appears to be of particular interest since it forms readily upon air oxidation of the thiol precursors in aqueous media. The subsequent thiol-disulfide exchange proceeding under thermodynamic equilibrium allows – by concentration changes or by the addition of a template – fine tuning of the product composition. We also study the abilities of the cyclodextrin duplexes to complex organic molecules within the elongated cavities. We have shown that α-cyclodextrin duplexes form complexes with α,ω-alkanediols in water with stability constants reaching up to 1010 M–1. 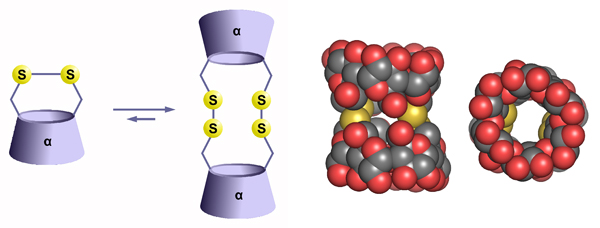 |
|
Cyclodextrin-flavin conjugates for organocatalytic oxidationsThe aim of the project is the design and the synthesis of flavin-based systems for enantioselective organocatalytic oxidations under environmentally benign conditions, in particular on organic sulfides and tertiary amines with regard to their chemoselectivity and enantioselectivity. In such systems, flavin-4a-hydroperoxide – formed in situ from the corresponding flavinium salt by the reaction with hydrogen peroxide or by the reaction of reduced flavinium salt with dioxygen – is the putative oxidizing species. Cyclodextrins are potentially attractive supporting scaffolds for the flavinium groups as they are known to form inclusion complexes with a broad range of organic guests in aqueous solutions and their cavities are inherently chiral. Thus, the cyclodextrin macrocycle attached to the flavinium group can help preorganize the substrate to the catalytic center and, at the same time, it can act as a chiral auxiliary allowing for enantioselective mode of the catalysis. The project is carried out in cooperation with Dr. Radek Cibulka, Institute of Chemical Technology, Prague. 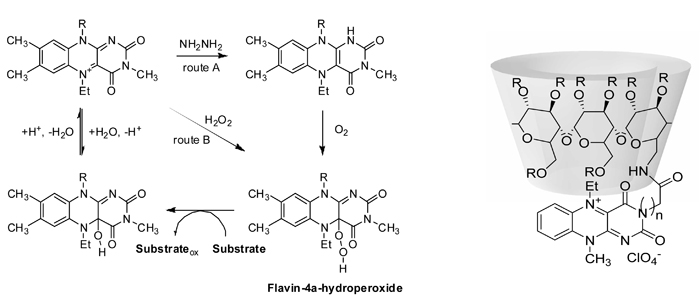 |
|
Synthesis of polyazamacrocycles by means of the [3+3] cyclocondensation | |
|
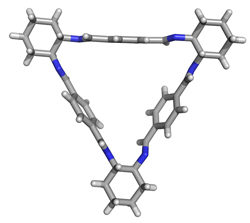
Our studies on the condensation reaction of enantiomerically pure trans-1,2-diaminocyclohexane with aromatic dialdehydes have revealed that the rigid rod-like dialdehydes give exclusively the products of the [3+3] cyclocondensation as thermodynamically the most stable products. The macrocyclic Schiff bases are obtained in excellent yield without employing a method of high dilution or a template. Currently, the cyclocondensation reaction of a series of rigid dialdehydes is elucidated with aim to find general scope and limitations of this highly effective synthetic approach to chiral polyazamacrocycles. Reversible character of the formation of Schiff bases would allow us to extend this synthetic strategy to the production of dynamic combinatorial libraries of chiral polyazamacrocycles as well as to study the effect of a template on the composition of the equilibrium mixture. |
|