2009
|
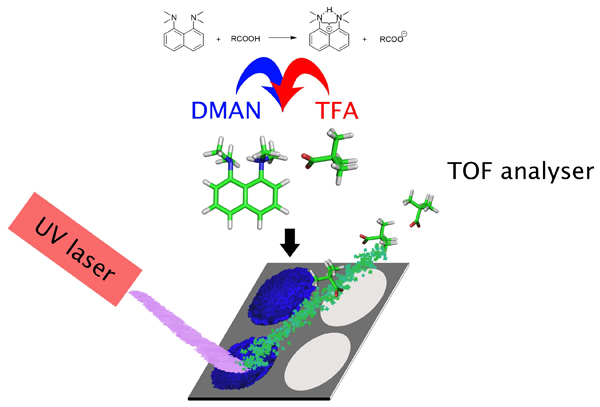
Acid-base-driven matrix-assisted mass spectrometry for targeted metabolomicsThe ability to charge huge biomolecules without breaking them apart has made matrix-assisted laser desorption/ionization (MALDI) mass spectrometry an indispensable tool for biomolecular analysis. Conventional, empirically selected matrices produce abundant matrix ion clusters in the low-mass region (<500 Da), hampering the application of MALDI-MS to metabolomics. An ionization mode of MAILD, a rational protocol for matrix selection based on Brönsted-Lowry acid-base theory and its application to metabolomics, biological screening/profiling/imaging, and clinical diagnostics is illustrated. Numerous metabolites, covering important metabolic pathways (Krebs' cycle, fatty acid and glucosinolate biosynthesis), were detected in extracts, biofluids, and/or in biological tissues (Arabidopsis thaliana, Drosophila melanogaster, Acyrthosiphon pisum, and human blood). This approach moves matrix selection from "black art" to rational design and sets a paradigm for small-molecule analysis via MALDI-MS.
In cooperation with: MPI for Chemical Ecology, Jena, Germany.
6-Hetaryl-7-deazapurine ribonucleosides - a novel type of nanomolar cytostaticsWithin the framework of our systematic study of biological activity of purine and deazapurine nucleosides we have discovered an exceptionally strong cytostatic effect of 6-hetaryl-7-deazapurine ribonucleosides. A large series of compounds of this type was prepared by cross-coupling reactions of 6-chloro-7-deazapurine nucleosides with hetarylboronic acids or hetarylstannanes. Their biological activity screening revealed that in particular compounds bearing a furyl or thienyl group in position 6 and hydrogen or fluorine in position 7 exert cytostatic effect against broad spectrum of tumor and leukemia cell lines at nanomolar concentrations (comparable to clinical cytostatics Gemcitabinem and Clofarabinem). The metabolism was also studied and their enzymatic phosphorylation to nucleoside triphosphates that inhibit RNA polymerases was detected. The most promising compound, 6-(2-thienyl)-7-fluor-7-deazapurin ribonucleoside, was selected as candidate which was submitted for preclinical in vivo tests in mice models at University Hospital in Olomouc. Preliminary results of toxicology and in vivo efficacy seem to be promising for further continuation of dveelopment of these novel cytostatics.

Nauš P., Hocek M.:
In cooperation with: Gilead Sciences, Inc. and Palacký University Olomouc.
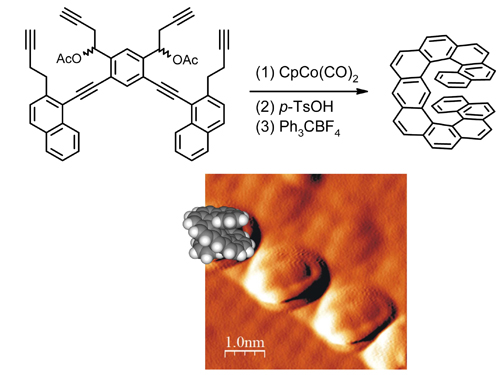
An organometallic route to long helicenesAlong with the recent progress in the development of advanced synthetic methods, the chemical community has witnessed an increasing interest in promising carbon-rich materials. Among them, helicenes are unique 3D aromatic systems that are inherently chiral and attractive for asymmetric catalysis, chiral recognition and material science. However, there have been only limited attempts at synthesizing long helicenes, which represent challenging targets. Here, we report on an organometallic approach to the derivatives of undecacyclic helicene, which is based on intramolecular [2+2+2] cycloisomerization of aromatic hexaynes under metal catalysis closing six new cycles of a helicene backbone in a single operation. The preparation of nonracemic compounds relied on racemate resolution or diastereoselective synthesis supported by quantum chemical (density functional theory) calculations. The fully aromatic [11]anthrahelicene was studied in detail including the measurement and theoretical calculation of its racemization barrier and its organization on the InSb(001) surface by STM. This research provides a strategy for the synthesis of long helical aromatics that inherently comprise two possible channels for charge transport: Along a π-conjugated pathway and across an intramolecularly π-π stacked aromatic scaffold.
Contacts: Dr. Ivo Starý [stary@uochb.cas.cz (+420) 220183315]. |