Projects
Chemistry of fluorinated phosphonatesWe have developed new methodologies for nucleophilic transfer of fluorine-containing groups using fluorinated phosphonates.
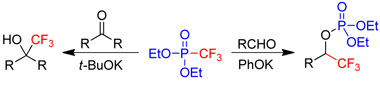
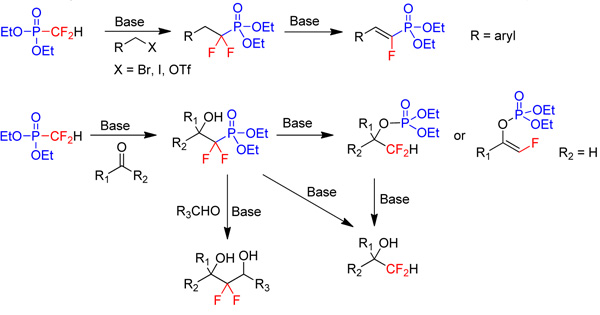
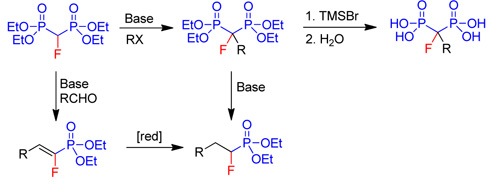
|
|
Nucleophilic and radical transfer of tetrafluoroethyl and tetrafluoroethylene groupsStarting from dibromotetrafluoroethane we have synthesized a variety of nucleophilic and radical sulfur-containing compounds, which are now under investigation as reagents for tetrafluoroethyl and tetrafluoroethylene group transfer.
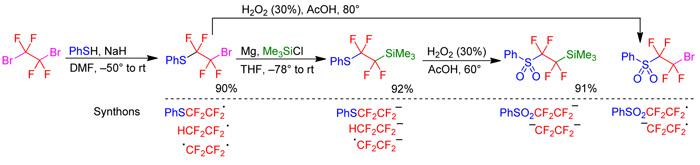
|
|
|
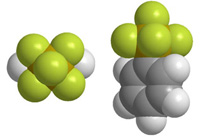
New methodologies for the preparation of (pentafluorosulfanyl)benzenesOrganic compounds with pentafluorosulfanyl (SF5) groups display a unique set of physicochemical properties. This includes extreme kinetic and hydrolytic stability, very strong electron acceptor capability, high lipophilicity with high SF5 electronegativity. A very high dipole moment can be achieved by the introduction of SF5 group without increasing molecular polarity. These properties make the pentafluorosulfanyl group an increasingly interesting structural motif for the design of bioactive compounds, including agrochemicals and pharmaceuticals as well as functional materials such as polymers or liquid crystals. However, access to SF5-containing compounds is very limited and their chemistry remains largely unexplored. In this project we are developing new methodologies towards substituted (pentafluorosulfanyl)benzenes. |
|
Biocatalysis in unnatural mediaIn this project, we exploit extremely inert and hydrophobic properties of perfluorinated hydrocarbons by employing them as reaction media in enzyme catalyzed processes. We are studying microbial Baeyer-Villiger oxidations of cyclic ketones to lactones performed in mostly perfluorocarbon medium or in aqueous-perflurocarbon liquid-liquid mixtures. Proposed experiments target the most important factors, that cause limitations in efficiencies of current processes, mainly limitation of oxygen transfer from the gas phase to the microorganism and inhibition of microorganism by substrate or product at certain concentrations. The use of perfluorocarbons in the reaction medium should also bring advantages in product separation, modifications in substrate and reaction scope and improvements in biocatalyst selectivity. |
|
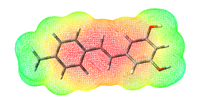
Synthesis of fluorinated derivatives of natural products and fluorine containing bioactive compoundsMany marketed drugs and agrochemicals contain one or more fluorine atoms in the molecule. This often brings improvements including better bioavailability and increased potency and lowered metabolic rate. We are working on the synthesis of new fluorinated derivatives of naturally occurring compounds with improved biological activity. |