Projects
Disulfide chemistrySome of the most important functionalities known to participate in biological chemistry are disulfides. One intriguing and biologically relevant reaction of disulfides is found in the pyruvate dehydrogenase system. There, lipoic acid, a naturally occurring disulfide, acts as an oxidant to transform pyruvate to acetylCoA through the action of thiamine pyrophosphate (TPP). We investigate the alternative approach based on a transition metal mediated reaction of aldehydes involving insertion of the low valent transition metal into the aldehydic carbon hydrogen bond followed by the interception of this intermediate with disulfide or its equivalent. Employing the same strategy on other substrates featuring the carbon hydrogen bond that can be activated by transition metals (such as aromatics, heteroaromatic compounds, terminal acetylenes, olefins) will extend a synthetic utility of this method.
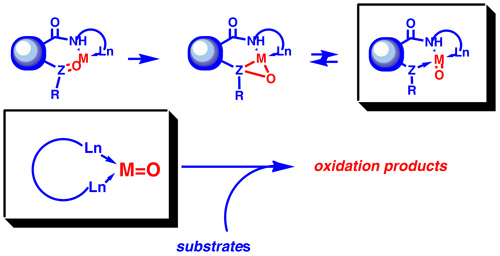
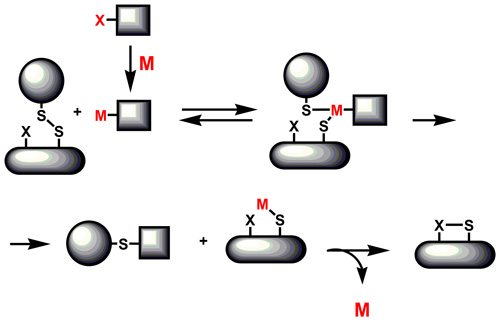 Replacing disulfides by sulfoxide functionality will further extend scope of the oxidative process. That could eventually lead to yet another variant of metal mediated oxidative chemistry. Here, we put emphasis on examining of various neighboring groups that may act as a tethered ligand which further activates reaction center.
Cross-coupling reactionsThe other project deals with cross-coupling reactions. Unlike halide-based leaving groups, used in this type of process, sulfur-based leaving group can be modified using the second sulfur atom valence. This allows for tailoring the leaving group according to the specific needs (desired selectivity, very polar solvents, very lipophilic solvents etc.); aspect unprecedented in synthetic organic chemistry. One of the most dramatic possibilities in the utilizing of the second sulfur valence is tailoring the leaving group to be specifically recognized by the reaction partner. 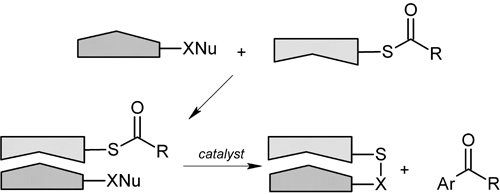 Although molecular recognition is a hot topic in modern science, application in synthetic organic chemistry is scarce, and it would certainly be, if successful, a valuable asset in the synthetic organic toolbox. The desired outcome could, once again, mean unprecedented selectivity of the cross-coupling reaction. |