Projects
 |
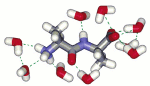
Spectroscopic and theoretical methods for non-covalent molecular interactionsExperimental and theoretical procedures for spectroscopy will be developed that will help to better understand molecular behavior in specific areas of organic chemistry and molecular biology. The computational tools will be based on combined ab initio and molecular dynamics. Application involve problems including relations between flexibility and reactivity of novel saccharide derivatives, resolving structure of nanoassemblies of peptides and porphyrins with a pharmaceutical potential, prion-derived peptides and complexes of nucleic acids and anti-cancer drugs. The project takes advantage of combining multiple techniques and expertise of scientists from different laboratories at the Institute of Chemical Technology, Charles University and the Institute of Organic Chemistry and Biochemistry. Also collaboration with the First Faculty of Medicine in Prague and laboratories abroad is part of the proposal. Eleven students will participate. |
 |
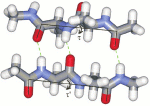
Conformational flexibility of peptides in solutionThe project aims at the dynamic aspects of peptide conformation in aqueous solution where peptide structural properties are most relevant to their biological role. In order to obtain solid physical knowledge about peptide structure and dynamics, we will proceed from simple model dipeptides to more complex systems. Experimentally the project is based mainly on Raman Optical Activity (ROA) and Raman scattering that can sense equilibrium distributions of rapidly fluctuating structures with parallel NMR experiments. Interpretation of Raman and ROA spectra will be based on advanced spectral simulations taking into account conformational flexibility of the studied systems as well as solvent effects. Potential energy surface will be mapped for simple models to get realistic estimates of their flexibility. Force fields and polarizability tensors of longer peptides will be constructed from short fragments by means of atomic tensor transfer. The ultimate goal is to fill the gap between knowledge about simple dipeptides and proteins and bring detailed interpretation of the peptide spectra. |
 |
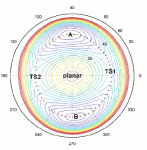
Conformational reorganizations in complex molecular systemsThe objective is a physically correct description of the conformational instability in complex molecular systems and exploring of its relevance for important chemical and biological processes. High priority is given to a combination of the theoretical and spectroscopical approaches. The aim will be achived by characterization of larger molecular reorganizations in terms of individual pathways for conformational changes pertaining to relatively small parts and chromophores of the bigger molecules. The emphasis is put on a detailed description of the purely quantum effects arising due to the tunneling motions opposed by surmountable energy barriers. |
 |
Chemical syntheses of fluorescence labeled mouse prion proteins using chemical ligations and investigation of their propertiesTransmissible spongiform encephalopathies (TSE) are fatal degenerative diseases of the central nervous system characterized by accumulation of abnormal form of cellular prion protein in the brain. In our project we are going to carry out chemical synthesis of Mouse Prion Protein (23-231) precursor of TSE causative agent - and some of its variants with and without fluorescence label at Lys, His and Arg residues. These fluorescence probes may serve as tools for a study of disease mechanisms, as well as, to explore the ability of prion protein to interact with cells and their components. Further progress of this area may contribute to development of early diagnostic test for TSE. In the frame of this project we will investigate conformations of prepared polypeptides and the prion proteins and study their properties. We will focus on their beta-structures responsible for toxic forms, and perform correlation of circular dichroism experiments with theoretical calculations. |