Projects
|
Our goal is to use heterocyclic chemistry as a tool to enter unexplored fields of molecular science. Pursued projects raise interesting and fundamental questions chemically. The synthetic strategies will be of broad utility for property generation and design, and will meet stringent criteria regarding economy of steps. By developing robust and streamlined synthetic schemes we plan to contribute significantly to the exciting search for novel concepts by designing, synthesizing, and testing e.g. organo- and metal catalysts, molecular devices and materials, molecular electronics components, and bioactive compounds.
Research interests
Helquats Read cover story: Adriaenssens et al. Chem. Eur. J. 2009 (15), 1072 We have developed a three-step synthesis of helquats, a novel class of small helical dications representing a missing structural link between helicenes and viologens. Bridging these areas, until now separate, is anticipated to open up unknown scientific territories. The parent helquat is a water-soluble, blue fluorophore and can be prepared by a unique metal-catalyzed [2+2+2] cycloisomerization in water under aerobic conditions.
Bio- and Air-tolerant Carbon-Carbon Bond Formations via Organometallic Ruthenium Catalysis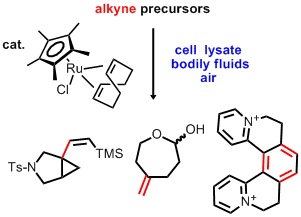 Although catalysis by organometallics is a powerful tool of organic synthesis, its use towards the formation of carbon-carbon bonds under mild conditions in presence of aerobic aqueous biological material has been widely neglected. Recently, Meggers and Streu effected the uncaging of amines from the corresponding allylcarbamates in living cells using commercially available catalyst [Cp*Ru(cod)Cl]. In other work directed towards organic synthesis in bio-media, the groups of Bertozzi and Sharpless have shown alkyne functionality to be significantly bioorthogonal. Inspired by these findings, we found that several known (Tetrahedron Lett. 2009, 4526) alkyne-based C-C bond formations catalyzed by commercially available organometallic ruthenium complex [Cp*Ru(cod)Cl], proceed in presence of complex aerobic aqueous media such as bodily fluids (e.g. human serum) or cell lysate (CCCC 2009, 1023). Thus Trost's alkene-alkyne coupling, Yamamoto's [2+2+2] cycloadditions, and Dixneuf's enyne-diazocompound fusion have been shown to be interesting candidates for studies directed to in situ synthesis for chemical biology. For details, see the free access article in the issue of CCCC dedicated to Dr. Alfred Bader on the occasion of his 85th birthday. |