| |
| |
| Original antiviral drugs developed in USA by Gilead Sciences: |
| |
|
|
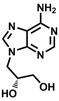
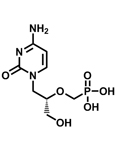
Vistide™ (cidofovir injection)
Used to treat opportunistic infections accompanying HIV/AIDS - strong effect against viruses at the origin of serious troubles in immunodepressed
patients: from peptic ulcers, through herpes simplex, smallpox, to viral retinitis. It was registered for clinical use in the U.S. in 1996.
|
|

|
Viread™ (tenofovir disoproxil fumarate)
The drug inhibits HIV multiplication. It cannot destroy the virus altogether, but delays AIDS development in HIV-infected patients.
Registered in the U.S. since 2001, it is one of the strongest drugs of its kind. This drug's use is authorised in many countries all over the world, including EU and Japan.
|
|

|
Hepsera™ (adefovir dipivoxil)
The drug was registered in the U.S. against viral B hepatitis in the autumn of 2002. Some 300 million people all over the world suffer of B hepatitis and one million die each year.
The drug's advantage is its virtual absence of side effects.
|
|

|
Truvada™ (emtricitabine and tenofovir disoproxil fumarate)
This drug's ambition is to be the most effective anti-AIDS pill to date - it helps HIV-infected patients to live longer. It is a combination of the Czech drug Viread
and the American Emtrivou (emtricitabine), registered in the U.S. under the name Truvada in 2004. Instead of thirteen pills that the patient was to take in a precise order
and at given times, one Truvada pill per day is enough. After 5 years of treatment, 65% of all treated patients show not HIV in their bloodstream as yet.
|
|

|
Atripla™ (efavirenz, emtricitabine and tenofovir disoproxil fumarate)
The first once-daily single tablet regimen (STR) for HIV-1 infection intended as a stand-alone therapy or in combination with other antiretrovirals. The product
combines Sustiva® (efavirenz), manufactured by Bristol-Myers Squibb Company, and Truvada® (emtricitabine and tenofovir disoproxil fumarate), manufactured by Gilead Sciences.
Atripla was cleared for marketing in the United States in July 2006. Atripla is indicated for use alone as a complete regimen or in combination with other antiretroviral agents
for the treatment of HIV-1 infection in adults.
|