Projects
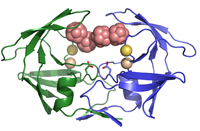 |
Structural studies of HIV proteaseIt has been almost 25 years since HIV was first identified as the causative agent of AIDS. Since 1996, when the first protease inhibitor (PI) was introduced, this enzyme became one of the primary targets for structure-based rational drug design leading to development of hundreds of inhibitory compounds, nine of which are currently approved for clinical use and several others are in the pipeline of pharmaceutical companies. Their effectiveness, however, is hampered by the emergence of drug-resistant variants. The major problem that limits the therapeutic efficiency of protease inhibitors (PIs) is drug resistance caused by extensive mutations in PR. |
|
Understanding PR resistance on structural level and development of new PIs acting by an alternative mode of inhibition, capable of inhibiting multidrug resistant species is thus essential for successful treatment of HIV-positive patients. Our recent contribution to the field of understanding HIV PR resistance and development of alternative PIs comprise several structural studies of the enzyme-inhibitor complexes. For example, in our search for novel structural types of unconventional protease inhibitors, we discovered and characterized a new class of potent HIV protease inhibitors, substituted metallacarboranes (Cígler et al., 2005, Kožíšek et al., 2008). The crystal structure revealed a unique binding mode, differing from clinical inhibitors used currently as anti-HIV drugs and also differing from all other PR inhibitory compounds for which the three-dimensional structure is known. We also elucidated the inhibition mechanism of these unusual compounds on a structural level and provided the model for molecular modeling studies and subsequent structure-based design of related inhibitors. Current structural studies are aimed at the structure-based drug optimization. |
|
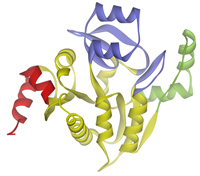 |
Structural studies of bacterial transcriptional repressorsIn many bacteria, the expression of the genes involved in the metabolism of secondary carbon sources is typically repressed in the presence of the most favorable nutrient. This mechanism of regulation, designated carbon catabolite repression, has been extensively studied in Bacillus subtilis. It requires participation of numerous specific repressors and activators controlling the level of transcription of catabolic genes. |
|
We have recently determined crystal structures of the effector binding domains of two transcriptional repressors from B. subtilis, CggR (central glycolytic genes regulator, PDB code 2OKG) and TreR (trehalose operon repressor, PDB code 2OGG). Structural analysis led to the identification of the effector binding sites and revealed novel insights into repressor oligomerization (Řezáčová et al., 2007; Řezáčová et al., 2008). In ongoing studies, we combine the X-ray crystallographic studies with complementary techniques, such as chemical cross-linking, isothermal titration calorimety, dynamic light scattering and analytical ultracentrifugation, to further characterize effector binding and modulation of the oligomeric states of these proteins. |
|
 |
European consortium: Targeting HIV integration co-factors, targeting cellular factors that act during nuclear import or integration of HIVHIV/AIDS remains a severe problem in the world, particularly in Africa. Despite improved access to treatment in poor communities due to efforts by governments and other stakeholders, current antiretroviral drugs are still unaffordable for many and the use of these drugs is associated with side effects. Moreover, incomplete suppression of HIV replication results in emergence of drug-resistant strains. Therefore, a continued research effort is required to develop more potent, cheaper and safer antivirals. |
|
The over-all objective of the European consortium is to develop novel treatment strategies by targeting cellular proteins required for HIV trafficking, nuclear import and integration. We predict that such drugs may elicit less drug resistance. To reach these objectives we formed a multidisciplinary European consortium composed of 3 biologists/ virologists, 3 medicinal chemists, 1 structural biologist, 1 pharmaceutical company and 1 virologist from South Africa. The project is coordinated by K. U. Leuven (Belgium). |
|