Hot stuff
|
Shining light on amide conjugate addition reactions - LiCl catalysis is the keyWhile earlier studies determined that stoichiometric amounts of LiCl inhibit conjugate addition reactions of lithium amides to α,β-unsaturated esters 1, it accelerates them in catalytic quantities! Moreover, it was known that LiCl also accelerates LDA-mediated deprotonation reactions, directed ortho-metalations. Collum and coworkers showed in a careful kinetic study that deaggregation to LDA monomers represents the rate limiting step before conjugate addition reactions of LDA to 1 occur as a fast step. In the strict absence of LiCl, the formed β-aminoester enolate 2 acts as a catalyst for its own formation, i. e. muted autocatalysis applies. When LiCl is present in catalytic quantities the formation of LDA monomers is promoted efficiently and the rate limiting step changes from deaggregation of LDA to the conjugate addition step of LDA monomer to 1. The authors note also the caveats of running reactions at -78 °C and comment very illustratively on complexity of LDA chemistry and its consequences for organic synthesis. No schadenfreude, please, but Freude.
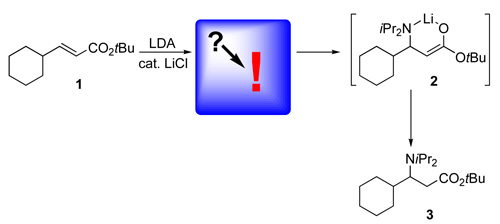 Y. Ma, A. C. Hoepker, L. Gupta, M. F. Faggin, D. B. Collum: J. Am. Chem. Soc. 2010, 132, 15610-15623
When is transition metal-free really transition metal-free?Recently, a number of reactions previously catalyzed by transition metal compounds was claimed to occur also under transition metal-free conditions. For many of these claims, it was found later, that even traces of transition metal contaminants in reagents might be responsible for noticeable catalytic effects. In other cases trace impurities of another transition metal in a given transition metal catalyst may be responsible for an observed result. Nichloas Leadbeater summarizes potential pitfalls (Nature Chemistry 2, 1007, 2010), and how to come around false claims in a short editorial, while Sun et al. (Nature Chemistry 2, 1044, 2010) demonstrate in a thorough study on the catalytic coupling of aryl halides 1 with benzene 2 to give biaryls 3 what has to be done to support a transition metal-free reaction.
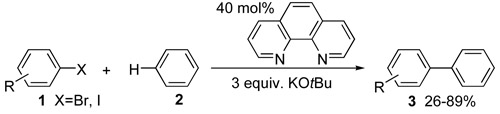 |