News
|
Direct connections between solution chemistry and gas-phase reactivity (Agrawal et al: Organometallics 29: 3979–3986, 2010 and Agrawal et al: Chemistry – An Asian Journal 5: 1667–1676, 2010.
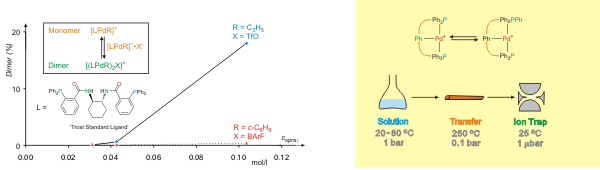 or two kind of palladium complexes, ESI-MS can provide direct insight into processes occurring in solution. Thus, the oligomerization of Pd(allyl)+ complexes of the Trost Standard Ligand, previously probed in solution by means of NMR, has also been observed in ESI mass spectra of the solutions in acetonitrile. With regard to the scrambling of phenyl groups between phosphine ligands and Pd-bound phenyl groups, ESI-MS can monitor the reaction occurring in solution upon heating, the reaction can also be induced by collisions of the mass-selected ions, and also heating in the interface between solution and the vacuum system can bring about the scrambling, thus leading from solution to the gas phase, back again, and in-between.
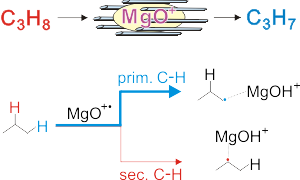
Preferential activation of primary C-H bonds takes place in the reaction of the gaseous MgO+ cation with small alkanes Schröder et al: Chemistry - A European Journal 16: 4110-4119, 2010. Detailed experimental and theoretical studies indicate that the origin of this potentially very useful kind of selectivity is due to a pronounced kinetic control in the attack of C-H bonds by MgO+ and its properties as oxygen-centered radical.
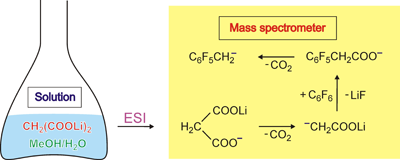
One of the mass spectrometric highlights from 2009 was reported by Meyer et al. (Meyeret al.: Angewandte Chemie - International Edition 48: 2934-2936, 2009.), who managed to generated the gas-phse equivalent of the dianion of acetic acid from aqueous solution. The key step to avoid hydrolysis of the lithium compound is its generation in the gas phase via decarboxlation of the corresponding malonate ion.
Recently, we published the generation of the ArCF22+ dication in the reaction of argon with the superelectrophile CF32+ (Journal of Physical Chemistry Letters 2010, 1, 358).
 The initial experiments were performed by two students from Warsaw (Poland) who visited the Prague team for a short summer internship. Any motivated student interested in such a visit is kindly invited to informally contact us via email. |