Projects
|
Antivirals
Systematic SAR studies are performed to identify novel types of antivirals, in particular compounds active against the resistant mutants that are insensitive to commonly used drugs. The aim of the project is also development of potential drug candidates for so far poorly treatable viral diseases (e.g. HIV, HCV). Activity of acyclic nucleoside phosphonates (ANPs) where the nucleobase is attached to a phosphonate moiety via a linker is based on structural similarity with naturally occurring metabolites. ANPs are excellent lead structures for drug design because of the high flexibility, absence of a labile glycosidic bond and enzymatic and chemical stability of the phosphonate moiety compared with the phosphate ester group. They can be transformed to pro-drugs to improve their pharmacological properties and to increase their ability to cross cell membranes and/or target the drug to specific tissue. Effects of various structural alterations of the nucleobase and linker on biological activity and toxicity are studied. The investigation of broad spectrum antiviral activities is performed in collaboration with Rega Institute, Katholic University Leuven (Belgium) and with Gilead Sciences (USA). |
|
Acyclic nucleoside phosphonates with antimalarial activity
The purine salvage enzyme hypoxanthine-guanine-xanthine phosphoribosyltransferase (HGXPRT) is essential for purine mononucleotides synthesis in malaria parasite Plasmodium falciparum (Pf). Humans possess two metabolic pathways, de novo and salvage, and hence are less reliant on the purine salvage pathway for survival. This provides a rationale for the design of inhibitors of PfHGXPRT which will arrest parasitemia and not be toxic to the human host. Acyclic nucleoside phosphonates (ANPs) are structural analogs of the nucleotide product of the enzyme reaction. Our aim is to design ANPs containing a purine base linked by various acyclic chains to a phosphonate group as potent and selective inhibitors of PfHGXPRT and antimalarial drug leads. The investigation of inhibitory activity of targeted ANPs is performed in collaboration with the laboratory of Dr. Luke W. Guddat, University of Queensland, Brisbane (Australia). |
|
Immunomodulating agents
|
|
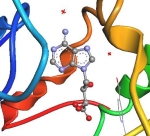
Inhibitors of Toxoplasma gondii S-adenosyl-L-homocysteine hydrolase
|

|
Inhibitors of Mycobacterium tuberculosis thymidylate kinase
|
| We cordially invite talented and hard-working organic chemistry students interested in medicinal chemistry to join our group and to participate in our projects. | |