Projects
 |
Development of prodrugs of acyclic nucleoside phosphonatesThe highly polar character of ANPs is responsible for their limited transport through the cell membrane and unfavorable pharmacological properties in general. Phosphonate based drugs can be absorbed by gastrointestinal tract in a limited scope which disqualify them for oral applications. In order to achieve oral bioavailability and intracellular delivery of acyclic nucleoside phosphonates their transformation to appropriate prodrug form is highly advisable. Our research in this area involves the following topics:
The project developed in collaboration with University of Southern California (Professor Ch. McKenna) is aimed to prodrugs releasing an active compound in target tissue together with a completely nontoxic and natural accompanying material as by-product (peptide). Appropriate structural modifications of peptides to form peptidomimetics open the way to circumvent metabolic enzymes and improve their oral bioavailability. [picture source: http://www.ncnr.nist.gov/programs/reflect/rp/biology/cell_membrane.html] |
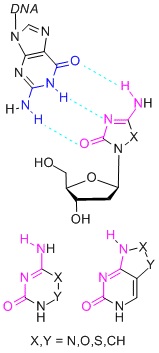 |
Novel cytosine mimics for epigenetically active nucleosidesRecently (2011) we identified the hypomethylating potential of 2’-deoxy-5,6-dihydro-5-azacytidine (DHDAC) hitherto considered inactive. SAR studies of DHDAC prodrugs and congeners are currently underway. The whole project is aimed at screening for alternative nucleobases with the cytosine base-pairing pattern, which are used for the design of nucleosides with potential gene silencing activity on the basis of inhibition of DNA-methyltranferases. |
 |
Thymidine phosphorylase inhibitorsThe project is focused to search for novel inhibitors of thymidine phosphorylase as potential anti-angiogenic agents for cancer chemotherapy. Syntheses falling within this topic involve 5-substituted pyrimidines, 5,6-disubstituted pyrimidines and corresponding ANPs. |
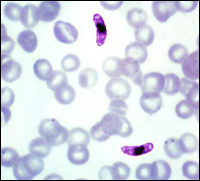 |
AntimalarialsThe project is based on rational design and syntheses of diverse guanine and hypoxanthine ANPs as inhibitors of hypoxanthine-guanine-xanthine phosphoribosyltransferase (HGXPRTase), a key enzyme of purine salvage pathway of malarial parasite Plasmodium falciparum. |
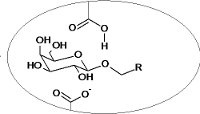 |
Enzymatic glycosylationsThe project is directed to enzymatic preparation of glycosides of biologically active acyclic nucleoside phosphonates. Transformation to glycosides improves solubility of compounds; moreover, interactions glycosides with lectines or specific hydrolysis of glycoside bond makes glycosylation an interesting implement for preparation of tissue specific prodrugs. |
 |
Antipox-virus active acyclic nucleoside phosphonates (ANPs)The common project being developed in collaboration with Gilead Sciences (USA) and Rega Institute in Leuven (Belgium) is aimed to investigation of ANPs as antipox-virus agents, finally targeted to variola virus as potential bioterrorist weapon. An indivisible part of the project is synthesis and pharmakokinetic studies of prodrugs of selected compounds.
|
 |
New structures for general biological screeningWe deal with syntheses of novel deazapurine nucleosides and nucleotides as potential antiviral and antitumor agents, fluorinated ANPs and 5-azacytidine congeners for epigenetic therapy of cancer. |