|

 |
The acronym HORIZOMS stands for "New Horizons in Mass Spectrometry", which is funded as an Advanced Grant of the European Research Council from 2009 to 2014. The aim of HORIZOMS is to bridge the wide gap between chemical reactions occurring in the gaseous or condensed phase at ambient or elevated temperatures and pressures on the one hand and sophisticated model studies of elementary reactions conducted in the highly diluted gas phase or single-crystal surfaces on the other. The key problem is that the model studies provide deep insight into the individual steps of chemical reactions, but these often have very limited relevance for real chemical processes occurring in the bulk. Therefore, the profound mechanistic knowledge achieved for elementary reactions often cannot be used to improve real chemical transformations. This dilemma is commonly referred to as "pressure gap".
|
|
The project HORIZOMS, in brief, attempts to connect the chemical and physical processes occurring under the extreme conditions of a mass spectrometer (ultrahigh vacuum, isolated molecules or atoms) with the complex processes occurring in real chemical transformations (synthesis, catalysis, molecular recognition etc.). To this end, various types of established methods of condensed-phase chemistry will be directly coupled with mass spectrometry.
In a more general sense, the aim of the entire project is to provide the foundations for a deeper understanding of chemical reactions and thereby contribute to more sustainable processes.
|
HORIZOMS tries to bridge this gap by various kinds of combination of modern mass spectrometric methods with preparative procedures in solution and condensed-phase measurements. In a nutshell: Reaction mixtures from the lab should be directly infused into a mass spec. This ambitious enterprise will be approached systematically in a stepwise manner. As the key interface between solution phase and gas-phase chemistry we will apply electrospray ionization.
The first steps, which already have been mastered in part, involve the investigation of solvation equilibria in the gas-phase ("microsolvation") and in real media. In the case of uranyl(VI) nitrate, UO2(NO3)2, for example, we managed to establish for the first time a semi-quantitative correlation (Fig. 1) between the concentration of the salt in aqueous solution and the peak patterns observed with mass spectrometry (N. Tsierkezos et al.: Inorg. Chem. 2009, 48, 6287–6296).

Fig. 1. Distribution of uranium cluster ions in MS for feed solutions of various concentrations.
For another case of salt solutions, which was subject to some debate in the literature (see: Y. Marcus, G. Hefter: Chem. Rev. 2006, 106, 4585-4621), i.e. the aqueous solutions of nickel perchlorate and nickel sulfate, we have recently obtained mass spectrometric data which may provide an explanation for the observed discrepancies. This project is still in progress and will be published in due course. Similar projects in the HORIZOMS team deal with the microsolvation of ammonium- and immonium ions (Fig. 2), the role of clustering in palladium catalysis, and the coordination chemistry of cadmium in the context of its biomobility.
 Fig. 2. Gas-phase infrared spectrum of the ion-pair complex [(Me4N)+(PF6)-[(Me4N)+].
Fig. 2. Gas-phase infrared spectrum of the ion-pair complex [(Me4N)+(PF6)-[(Me4N)+].
The gas-phase IR spectrum of the mass-selected ion was recorded at the European multi-user facility CLIO (Orsay, France).
Parallel to these studies on ion solvation, we investigate the on-line coupling of chemical reactions with mass spectrometry using electrospray ionization as an interface. Recently, for example, we investigated the course of reaction of a Cu(I)-mediated C-S coupling (Scheme 1) by directly spraying the reaction mixture to the mass spectrometer followed by MS/MS experiments (J. Šrogl et al.: Chem. Commun. 2009, 3463-3465). The mass spectrometric data demonstrate that at least in this particular case, the reaction can occur via mononuclear copper complexes without involving radical intermediates.

Scheme 1. Cu(I) catalyzed disproportionation of bisiminodisulfides.
In the forthcoming steps of HORIZOMS, we will implement various combinations of condensed-phase methods with mass spectrometric studies. Given the preconditions of both regimes, electrochemical methods and in particular electron paramagnetic resonance (EPR) appear particularly promising because both methods are very sensitive and the typical concentrations are similar to those used in electrospray ionization. Another important novelty is the implementation of the ion-mobility technique, a mass-spectrometric method which can distinguish ions not only by mass but also by their shapes.
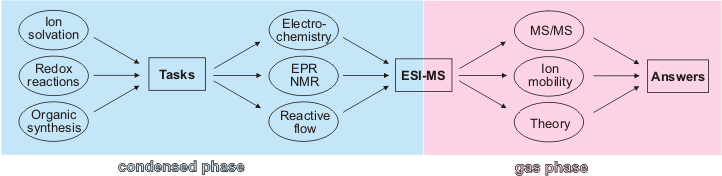
Topics of research
-
Ion solvation. Dissolved salts are ubiquitous in daily life and are of immanent importance in industry. Ion solvation is hence a topic of dense research, but yet there exists a huge gap between macroscopic models and the microscopic understanding of ion solvation. Thus, some central concepts in ion solvation only recently have been questioned in their foundations. In this context, also extension of electrospray ionization towards superacidic media will be attempted.
-
Redox intermediates. Ever since times of alchemy, reduction- and oxidation reactions (redox processes) are key themes in chemical research with many implications for other sciences, biology in particular. Nevertheless, redox intermediates are still difficult to characterize, and the development of innovative methods for their detection is thus highly beneficial for research in chemistry and related fields.
-
Reaction mechanisms in metal catalysis. Metal catalysts are essential in many chemical and technical processes, but their precise mode of action is difficult to investigate. The project thus aims to combine solution- and gas-phase studies in order to offer new, more facile tools for the elucidation of organometallic reaction mechanisms.
|





