2014
|
Observation of Paramagnetic Raman Optical Activity of Nitrogen DioxideRotation of the plane of linearly polarized light when interacting with asymmetric molecules has been attracting people’s attention since the time of Luis Pasteur (1848), because of the underlying physical principles and relation to biological compounds. Interestingly, almost at the same time, magneto-optics phenomena were discovered, such as the Faraday Effect. Until now, several other techniques were developed and are routinely used in laboratories, with or without the magnet, like circular dichroism or Raman optical activity. In this study, we reported a new flavor of such magneto-optic spectroscopy, paramagnetic Raman optical activity of gases. This was considered difficult because of the low signal. We succeeded because of a construction of the magnetic cell, a large signal of the NO2 molecule, and a detailed theoretical analysis. The results suggest that the technique can bring about unique information about molecular properties, and may be even usable for a characterization of industrial gases.

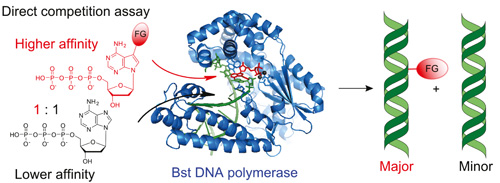
DNA polymerases preferentially synthesize artificial modified DNA even in presence of natural substratesScientists from the Joint Laboratory of Bioorganic and Medicinal Chemistry of Nucleic Acids of the Institute of Organic Chemistry and Biochemistry AS CR and Faculty of Science of the Charles University (group of Prof. Hocek) discovered a whole class of artificial labelled nucleoside triphosphates that are surprisingly much better substrates for DNA polymerases than the natural nucleotide (dATP) hence the enzymes preferentially synthesize artificial modified DNA and explained the mechanism of this unusual activity. This finding not only significantly contributes to the knowledge of mechanism of DNA replication but also paves the way to enzymatic synthesis of modified nucleic acids for applications in diagnostics and chemical biology.
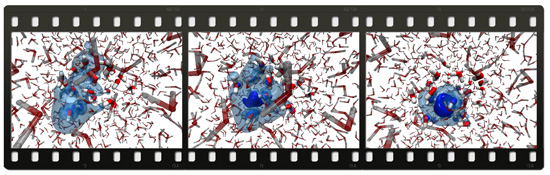
How is a "wet" electron formed ?Everybody who ever put salt in a soup knows how ions dissolve in an aqueous environment. How does, however, electron dissolve in water? Does this negatively charged elementary particle dissolve similarly to a chloride anion from kitchen salt or do quantum mechanical effects kick in and ensure a completely different dissolution scenario? These questions not only touch the frontier between classical and quantum mechanics (investigations and modeling of which has been rewarded by the Chemistry Nobel Prize in 2012) but also have practical implications in radiation chemistry. Formation and dissolution of electrons in water and subsequent reactions are important for understanding radiation cancer therapy, as well as chemical processes taking place during nuclear waste storage. Calculations combining classical and quantum mechanics, carried out at the Institute of Organic Chemistry and Biochemistry AS CR, together with ultrafast terahertz laser experiments, performed at the University of Zurich in Switzerland, give an answer to fundamental questions concerning formation of the hydrated electron. It turns out that electron is formed in water via photoionization as a delocalized quantum wave which, however, within a picosecond (i.e. a millionth of a millionth of a second) contracts to a roughly spherical object with a diameter of a quarter of a nanometer. One says that seeing is believing. The study of Czech and Swiss chemists shows directly how a nascent electron looks like in water and how extremely rapidly it dissolves.
Structural and biochemical study of interactions mediating formation of immature and mature retroviral particlesRetroviruses such as Human immunodeficiency virus type 1 (HIV-1) are of great medical importance. Retroviral assembly proceeds in two stages. First, the viral Gag polyprotein assembles into a hexameric protein lattice at the plasma membrane of the infected cell, inducing budding and release of an immature particle. During second stage, Gag is cleaved by the viral protease, leading to internal rearrangement of the virus into the mature, infectious form. Using Mason-Pfizer monkey virus (M-PMV) as a model retrovirus, we studied the structural organization of retroviral particles and interactions mediating the formation of both immature as well as mature particles. We provided biochemical and structural data confirming the general relevance of a short segment of the structural polyprotein Gag for retrovirus assembly and infectivity (Strohalmová-Bohmová et al., 2014). Combining biochemical and structural NMR analyses, we identified a network of supportive interactions that stabilize the M-PMV capsid protein (CA) in mature conformation (Obr et al., 2014). We also contributed to resolve the structure of the capsid lattice within intact immature HIV-1 and M-PMV particles at subnanometre resolution using cryo-electron tomography and sub-tomogram averaging methods. The resulting model reveals tertiary and quaternary structural interactions that mediate HIV-1 and M-PMV assembly. Comparison the immature HIV-1 with M-PMV structures reveals that retroviral capsid proteins, while having conserved tertiary structures, adopt different quaternary arrangements (Schur et al., 2014).
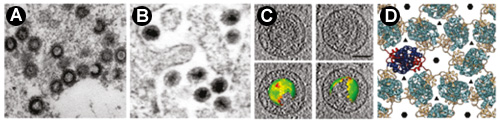
Obr M., Hadravová R., Doležal M., Křížová I., Papoušková V., Žídek L., Hrabal H., Ruml T., Rumlová M.:
Schur F., Hagen W., Rumlová M., Ruml T., Müller B., Kraeusslich H.-G., Briggs J.,:
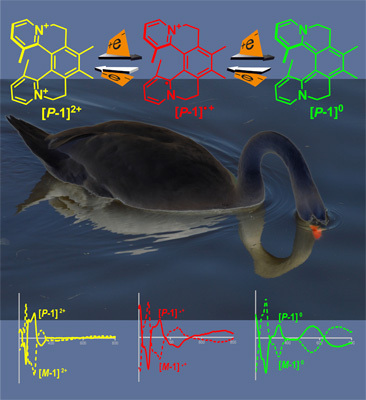
Intense Chiroptical Switching in a Dicationic Helicene-Like Derivative: Exploration of a Viologen-Type Redox Manifold of a Non-Racemic HelquatThis report documents a new level of control over the chiroptical properties of helical systems, known as helquats. By simply altering the molecules’ redox states, enantiopure helquats, while retaining their overall shape, undergo a profound change in their electronic state and thus sizable changes in their electronic circular dichroism spectra at certain wavelengths, which is unique for a chiroptical switch. Furthermore, this helically chiral system features the most intense chiroptical switch response documented in the field of helicenoids. This unprecedented example of a "chiro-switch" may lead to the development of new classes of optical switches and light modulators and contribute to the emerging field of chiral organic conducting systems.
|