Our research team aims to advance science in the field of optical biosensors, and furthermore, develop novel optical biosensor technologies for applications in biomolecular research and bioanalytics. This highly multidisciplinary research requires a concerted effort in multiple areas, including: research into photonic and plasmonic (nano)structures; the development of functional biomolecular systems; and the development of sensor instruments. The instruments developed by our research team are used for a variety of applications, among other fields, the investigation of biomolecules and their interactions, and the detection of chemical and biological substances.

Relevant publications:
- J. Homola: Surface plasmon resonance sensors for detection of chemical and biological species, Chemical Reviews, 108, 462-493 (2008). link
- J. Homola (editor): Surface plasmon resonance based sensors, Springer, 2006. link
Surface plasmons and surface plasmon-related phenomena on photonic (nano)structures
We carry out theoretical and experimental research to expand the understanding of optical phenomena in photonic (nano)structures, in particular, in (nano)structures supporting various types of surface plasmons, with a focus on their role in optical biosensing based on Surface Plasmon Resonance (SPR) and Surface-Enhanced Raman Scattering (SERS).






Relevant publications:
- M. Vala, J. Homola: Multiple beam interference lithography: A tool for rapid fabrication of plasmonic arrays of arbitrary shaped nanomotifs, Optics Express, 24, 15656-15665 (2016).
- B. Špačková, P. Lebrušková, H. Šípová, P. Kwiecien, I. Richter, J. Homola: Ambiguous refractive index sensitivity of Fano resonance on an array of gold nanoparticles, Plasmonics, 9: 729 (2014) link
- B. Špačková and J. Homola: Sensing properties of lattice resonances of 2D metal nanoparticle arrays: An analytical model, Optics Express 21(22), 27490 - 27502 (2013) link
- M. Piliarik, H. Šípová, P. Kvasnička, N. Galler, J. R. Krenn, J. Homola: High-resolution biosensor based on localized surface plasmons, Optics Express, 20, 672–680 (2012). link
- M. Piliarik, P. Kvasnička, N. Galler, J. R. Krenn, J. Homola: Local refractive index sensitivity of plasmonic nanoparticles, Optics Express, 19, 9213–9220 (2011). link
Functional biomolecular systems for affinity biosensors
We investigate approaches to interface inorganic photonic (nano)structures with biomolecules. Specifically, we look to control the placement of the biomolecules on the photonic (nano)structures, and furthermore, to optimize the properties of the photonic (nano)structures functionalized with biomolecules with respect to their interactions with biological media.




Relevant publications:
- H. Vaisocherová, V. Ševců, P. Adam, B. Špačková, A. S. Pereira, C. Rodriguez-Emmenegger, T. Riedel, E. Brynda, J. Homola: Functionalized ultra-low fouling carboxy- and hydroxy-functional surface platforms and their fouling resistance from undiluted biological media and biorecognition capability, Biosensors and Bioelectronics, 51, 150–157 (2014). link
- M. Piliarik, M. Bocková, J. Homola: Surface plasmon resonance biosensor for parallelized detection of protein biomarkers in diluted blood plasma, Biosensors and Bioelectronics, 26, 1656-1661 (2010). link
Measurement methods and sensor instrumentation
We research optical measurement techniques, including measurement principles, instrumentation, and data processing approaches, to create optical systems for high-performance optical biosensors. We focus on the research and development of compact systems.



Relevant publications:
- M. Vala, K. Chadt, M. Piliarik, J. Homola: High-performance compact SPR sensor for multi-analyte sensing, Sensors and Actuators B, 148, 544-549 (2010). link
- T. Špringer, M. Piliarik, J. Homola: Surface plasmon resonance sensor with dispersionless microfluidics for direct detection of nucleic acids at the low femtomole level, Sensors and Actuators B, 145, 588-591 (2010). link
- M. Piliarik; M. Vala; I. Tichý; J. Homola: Compact and low-cost biosensor based on novel approach to spectroscopy of surface plasmons, Biosensors & Bioelectronics, 24 3430–3435 (2009). link
- M. Piliarik, L. Párová, J. Homola: High-throughput SPR sensor for food safety, Biosensors & Bioelectronics, 24, 1399-1404 (2009). link
Mikrofluidics and transport phenomenon
We investigate microfluidic methods to improve the performance of optical biosensors, focusing on the design and optimization of low-cost and practical flow cells for everyday use. In this process we examine the interplay between the physics associated with the sensor design and the role of convection, diffusion, and chemical reactions.

Mixing of the fluid in the sensing chamber is useful for sensors suffering from mass transfer limitations; the stirring of fluid within each channel increases the rate of analyte capture.

A 5-channel flow cell including mixing grooves.

SPR sensor response of the detection of ssDNA comparing an unmixed channel with a mixed channel, which displays a 70% improvement in the initial binding rate (left). The improvement of a sensor using mixed channels is dependent on the Péclet number, the experimental improvement compares well with theory (right).

Analyte transport to an array of LSP supporting nanoparticles. Changes to the design of the array can have profound impacts on the rate of analyte transport, which is important for the design of an optimal sensing platform.
Relevant Publications:
- Lynn, N. S.; Bocková, M.; Adam, P.; Homola, J. Biosensor Enhancement Using Grooved Micromixers: Part II, Experimental Studies. Analytical Chemistry 2015, 87, 5524-5530.
- Lynn, N. S.; Homola, J. Biosensor Enhancement Using Grooved Micromixers: Part I, Numerical Studies. Analytical Chemistry 2015, 87, 5516-5523.
- Lynn, N. S.; Martinez-Lopez, J. I.; Bocková, M.; Adam, P.; Coello, V.; Siller, H. R.; Homola, J. Biosensing enhancement using passive mixing structures for microarray-based sensors. Biosensors & Bioelectronics 2014, 54, 506-514.
- Lynn, N. S.; Šípová, H.; Adam, P.; Homola, J. Enhancement of affinity-based biosensors: effect of sensing chamber geometry on sensitivity. Lab on a Chip 2013, 13, 1413-1421.
- Špringer, T.; Piliarik, M.; Homola, J. Surface plasmon resonance sensor with dispersionless microfluidics for direct detection of nucleic acids at the low femtomole level. Sensor Actuat B-Chem 2010, 145, 588-591.
Optical biosensors for investigation of biomolecules and their interactions
We pursue optical biosensor application for the study of biomolecules and their interactions, for example, interactions of nucleic acids, protein-protein interactions and studies of enzymatic activity. This effort comprises, in particular, the development of novel experimental methodologies and data analysis tools.

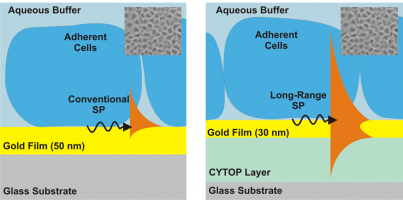

Relevant publications:
- H. Šípová, T. Špringer, D. Rejman, O. Šimák, M. Petrová, P. Novák, Š. Rosenbergová et al.: 5′-O-Methylphosphonate nucleic acids-new modified DNAs that increase the Escherichia coli RNase H cleavage rate of hybrid duplexes, Nucleic acids research (2014): Available online. link
- M. Vala, R. Robelek, M. Bocková, J. Wegener, J. Homola: Real-time label-free monitoring of the cellular response to osmotic stress using conventional and long-range surface plasmons, Biosensors and Bioelectronics, 40, 417-421 (2013). link
- T. Špringer, H. Šípová, H. Vaisocherová, J. Štepánek, J. Homola: Shielding effect of monovalent and divalent cations on solid-phase DNA hybridization: surface plasmon resonance study, Nucleic Acids Research, 38 , 7343-7351 (2010). link
- H. Šípová, H. Vaisocherová, J. Štěpánek, and J. Homola: A dual surface plasmon resonance assay for the determination of ribonuclease H activity, Biosensors & Bioelectronics, 26, 1605-11 (2010). link
Optical biosensors for detection of chemical and biological substances
We develop novel biosensors for the rapid and sensitive detection of chemical and biological substances relevant to medical diagnostics (disease biomarkers, e.g. molecular biomarkers of cancers, Alzheimer’s disease, organ damage, etc. ), environmental monitoring (pollutants and contaminants, e.g. atrazine, bisphenol A, benzo(a)pyren, nonylfenol, etc. ), and food safety (pathogens and toxins, e.g. Escherichia coli, Salmonella enterica, Lysteria monocytogenes, Staphylococcal enterotoxins, tetrodotoxin, etc.).



Relevant publications:
- H. Vaisocherová-Lísalová, I. Víšová, M. L. Ermini, T. Špringer, X. Song, J. Mrázek, J. Lamačová, N. S. Lynn Jr., P. Šedivák, and J. Homola: Low-fouling surface plasmon resonance biosensor for multi-step detection of foodborne bacterial pathogens in complex food samples, Biosensors and Bioelectronics, 80, 84–90 (2016).
- T. Špringer, M. Bocková, J. Homola: Label-free biosensing in complex media: a referencing approach, Analytical Chemistry, 85, 5637–5640, (2013). link
- T. Špringer, J. Homola: Biofunctionalized gold nanoparticles for SPR-biosensor-based detection of CEA in blood plasma, Analytical and Bioanalytical Chemistry, 404, 2869-2875 (2012). link
- H. Šípová, S. Zhang, A.M. Dudley, D. Galas, K. Wang, J. Homola: Surface plasmon resonance biosensor for rapid label-free detection of microribonucleic acid at subfemtomole level, Analytical Chemistry, 82, 10110-10115 (2010). link
- K. Hegnerová, J. Homola: Surface plasmon resonance sensor for detection of bisphenol A in drinking water, Sensors and Actuators B, 151, 177-179 (2010). link