Projects
|
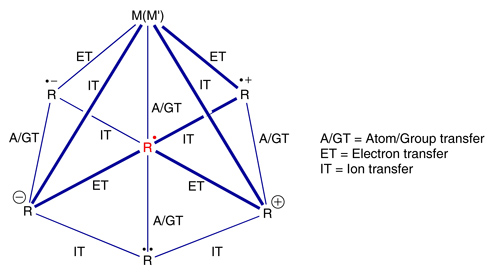
Development of Electron-Transfer-Induced Reaction SequencesOne of our major interests is the incorporation of selective electron transfer steps into tandem reactions. This allows a switch of reactive intermediates during sequential domino processes. In this way, the complementary reactivity of different intermediates, such as carbanions, radicals, carbocations, and carbenes can be used in one synthetic sequence. Figure bellow shows the general interconversion opportunities and the general reaction steps connecting the individual reactive intermediates. In principle, this allows unlimited opportunities for the design of new tandem sequences. Given that pericyclic and transition metal-catalyzed processes may also be incorporated into these sequences, the usefulness of domino processes is potentiated.
Total Synthesis of Natural Products & Analogs and Their Biological InvestigationsCyclic lipid metabolites beyond the prostaglandins are one of our major interests in the area of natural products chemistry. These metabolites are ubiquitous in nature. Among them are phytoprostanes in plants or isoprostanes, neuroprostanes and isofurans in humans and animals. These natural products are formed as regio- and stereoisomeric mixtures by free radical-initiated reactions under oxidative conditions in tissues from membrane-bound polyunsaturated fatty acids. In humans, isoprostanes are related to many diseases and their quantification represents today the "gold standard" for the quantification of oxidative stress. We are interested in the total synthesis of these natural products since it represents the only reliable method to access individual members and to study their biological profile. Other areas of interest are the total synthesis of biological active terpenes, lignans, and alkaloids. |