Projects
Mechanisms of action of nucleoside / nucleotide analogs and mechanisms of resistance |
|
 |
In the long-term, this project mainly aims to elucidate molecular basis of the antiproliferative activity of newly synthesized cytotoxic compounds. It starts by answering the questions whether and how the compounds are taken up by the cells, how they are metabolized and what are their active metabolites. We describe the apoptotic effects of such compounds as well as the effect on cell cycle distribution. We also follow the effect of the compounds on protein kinase- or caspase-mediated signaling, role of mitochondria, membrane 'death receptors' and interactions with telomeres / telomerase. Currently we focus on the metabolism and mode of action of novel carbocyclic nucleosides with activity against some RNA viruses and cytostatic activity. The knowledge of a compound's metabolism and mechanism of action allows us to identify cellular sites where the acquired resistance might originate from and give us an idea how to overcome this phenomenon. In resistant cells we study expression and function of membrane transporters of MRP and MDR class (e.g. P-glycoprotein), phosphorylation activity of key kinases and we also evaluate the sensitivity of resistant cells towards other commonly used cytostatics i.e. the ability of a compound to induce multidrug resistance. |
DNA methylation (epigenetics) |
|
 |
Epigenetics studies changes in gene expression caused by mechanisms that do not involve changes in DNA sequence. One of the major epigenetic mechanisms is represented by DNA methylation. Hypermethylation of 'CpG islands' located within the regulatory sequences of tumor suppressor genes frequently causes their silencing, which may lead to carcinogenesis. Restoring aberrant DNA methylation pattern with use of hypomethylating agents is expected to be more effective and less toxic method of cancer treatment compared to standard chemotherapy. Therefore, searching for new hypomethylating agents and reliable detecting methods is a top-priority task. |
Antiangiogenic effects of the compounds |
|
 |
Angiogenesis refers to new blood vessel formation based on existing vasculature. It is a complex, multi-step process that is influenced by a number of angiogenesis mediators (extracellular matrix components, growth factors, integrins, cytokines and enzymes) with inhibitory or stimulatory function. Angiogenesis dysregulation results in a number of pathological conditions such as rheumatoid arthritis or diabetic neuropathy. Last but not least, increased angiogenesis is necessary for the growth of solid tumors and their ability to form metastases. Traditionally we are dedicated to the identification of new thymidine phosphorylase (TP) inhibitors. This enzyme catalyzes the phosphorolysis of thymidine to thymine and 2'-deoxyribose-1-phosphate, which acts as chemoattractant. Angiogenic and antiapoptotic effects of TP (previously called PD-ECGF) are well known. Several 5-substituted 6-chlorouracil derivatives exerted a marked effect on human recombinant TP as well as on TP isolated from human placenta (Ki in submicromolar range, Nencka et al., 2007). Apart from TP inhibitors we also study the effect of selected compounds on other angiogenic factors such as VEGF. Currently we aim to set up new in vitro models for angiogenesis evaluation in cellular systems (endothelial cell migration and endothelial tube formation assays). |
Compounds influencing cAMP cellular signalisation |
|
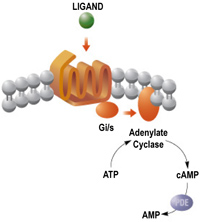 |
cAMP represents an adenosine triphosphate (ATP) derivative employed by most cells as an intracellular second messenger. It is formed from ATP by the catalytic activity of adenylate cyclases and decomposed by phosphodiesterases to 5'-AMP. Adenylate cyclase pathway generally consists of three structural parts: G-protein, G-protein-coupled receptor (GPCR) and adenylate cyclase itself. GPCR represent an important target for drug development (40-50% of all pharmaceuticals target GPCR). In our laboratory, we search for the ways of influencing intracellular cAMP levels by new analogs of nucleosides, nucleotides or nucleobases, particularly through the inhibition of adenylate cyclases - either cellular or exogenous (bacterial). We look for the compounds with implications in the therapy of some infectious and cardiovacular diseases. |
Novel xanthine oxidase inhibitors |
|
 |
Xanthine oxidoreductase (XOR) is a key enzyme of purine catabolic pathway, which catalyzes the oxidation of hypoxanthine to xanthine and finally to uric acid. Under some pathological conditions serum uric acid is elevated (hyperuricemia) and may result in the precipitation of uric acid crystals and the development of gout. In recent years, XOR has been linked with the increased generation of reactive oxygen species (ROS) contributing to the development of certain cardio-vascular disorders. Allopurinol, the competitive inhibitor of XOR, has been the drug of first choice in treatment of hyperuricemia and gout for several decades. However, due to its relatively low efficiency and poor ability to prevent XOR-related ROS generation, the search for new inhibitors continues. Currently we focus on identification of novel purine XOR inhibitors with the potential to provide an effective alternative to allopurinol. Inhibitors revealed in the primary screening as the most effective are subjected to kinetic studies and they are co-crystallized with XOR in order to clarify the inhibitory mechanism (in cooperation with the team of Structural Biology). |