2006
|
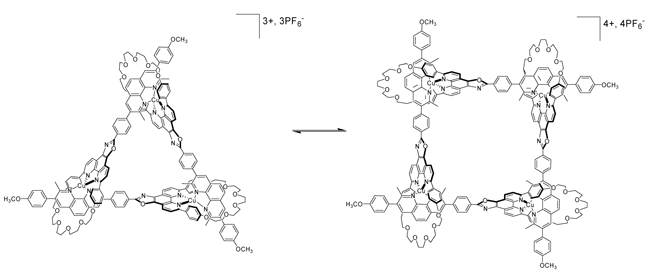
Copper(I)-Directed Formation of a Cyclic Pseudo-Rotaxane Tetramer and Its Trimer HomologueThe article describes the first synthesis of pseudorotaxanes with novel topology: the addition of stoichiometric amounts of copper(I) to the newly designed ligand affords cyclic pseudorotaxanes. The tetramer is the major product in concentrated solutions whereas the trimeric complex prevails in more diluted solutions (see scheme). Both homologous species can be interchanged via a slow equilibrium by modifying the solution concentration.
Kraus T., Buděšínský M. , Cvačka J. , Sauvage J.-P.:
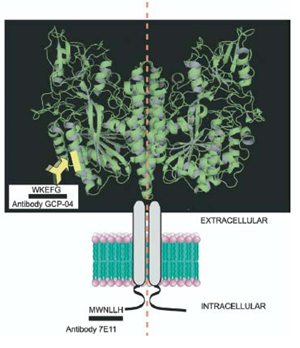
Recombinant Glutamate Carboxypeptidase II from Human BrainProteases are group of enzymes capable to cleave the peptide bond. These molecules play pivotal role in the regulation of number of biological processes. One of important proteases found in various human and animal tissues is glutamate carboxypeptidase II (GCP-II). In human brain this enzyme cleaves a neurotransmitter NAAG, liberating thus amino acid glutamate, which plays an important role in a number of pathologic conditions, such as brain stroke neuronal damage, diabetic neuropathy, Alzheimer disease etc. The animal experiments confirmed that GCP-II indeed is an important pharmaceutical target for the development of new neuroprotective drugs. In the IOCB we succeded in preparation of the recombinant GCP-II by expression in insect cells, characterise it enzymologically, develop novel sensitive monoclonal antibodies and, in collaboration with a German team, to solve the three-dimensional structure of the enzyme. The structure of this important pharmaceutical target will be used for the rational design of novel neuroprotective drugs based on GCP-II inhibition.
Mesters J.R., Bařinka C., Li W., Tsukamoto T., Majer P., Slusher B.S., Konvalinka J., Hilgenfeld R.:
Šácha P., Zámečník J., Bařinka C., Hlouchová K., Vícha A., Mlčochová P., Hilgert I., Eckschlager T., Konvalinka J.:
Hlouchová K., Bařinka C., Klusák V., Šácha P., Mlčochová P., Majer P., Rulíšek L., Konvalinka J.:
Quantification and Rationalization of the Higher Affinity of Sodium Over Potassium to Protein SurfaceFor a series of different proteins, including a structural protein, enzyme, inhibitor, protein marker, and a charge transfer system, we have quantified the higher affinity of Na+ over K+ to the protein surface by means of molecular dynamics simulations and conductivity measurements. Both approaches show that sodium binds at least twice as strongly to the protein surface as potassium, this effect being presentin all proteins under study. Different parts of the protein exterior are responsible to a varying degree for the highersurface affinity of sodium, with the charged carboxylicgroups of aspartate and glutamate playing the most importantrole. Local ion pairing is, therefore, the key to the surfacepreference of sodium over potassium, which is further demonstrated and quanified by simulations of glutamate and aspartate in the form of isolated amino acids, as well as short oligopeptides. As a matter of fact, the effect is already present at the level of preferential pairing of the smallest carboxylate anions, formate or acetate, with Na+ vs. K+, as shown by molecular dynamics and ab initio quantum chemical calculations. By quantifying and rationalizing the higher preference of sodium over potassium to protein surface the present study opens a way to molecular understanding of many ion-specific (Hofmeister) phenomena involving protein interactions in salt solutions. The stronger binding of Na+ to protein surface may also be one of the reasons why sodium has to be pumped out of the cell.
Higher affinity of sodium (green) over potassium (blue) to protein surface. Snapshot from molecular dynamics simulation of ribonuclease A and statistically averaged ion density distribution in the vicinity of different proteins.
Vrbka L., Vondrášek J., Jagoda-Cwiklik B., Vácha R., Jungwirth P.:
|