Projekty
Role of T-type Calcium Channels in Synaptic PhysiologyIt is established that presynaptic increase in T-type Ca2+ channel activity in primary nociceptive neurons contributes for the development of painful neuropathy including peripheral diabetic pain. However, the implication of T-type channel in synaptic activity remains unknown. There is increasing evidence that T-type Ca2+ channels, besides contributing to neuronal electrical activity, may also participate to the vesicular release of neurotransmitters at the presynaptic nerve terminals, and interestingly enough, we have recently shown that T-type channels form functional signaling complexes with some of the presynaptic proteins of the vesicular release machinery (SNARE proteins, for instance syntaxin-1A and SNAP-25). We are characterizing the biochemical coupling of T-type channels with SNARE proteins and investigate the functional importance of T-type channel / SNARE complexes in the synaptic physiology of nociceptive neurons to elucidate their role in pain transmission. |
|
|
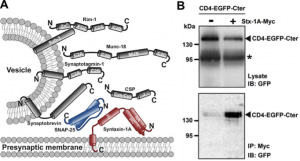
Illustrative citation: SNARE proteins interact within the C-terminal domain of Ca(v)3.2 T-type channel. A, Schematic representation of the presynaptic vesicular release machinery. B, Co-immonoprecipitation of CD4-EGFP-Ca(v)3.2Cter fusion protein with myc-tagged syntaxin-1A expressed in tsA-201 cells.*, possible degradation of the CD4-EGFP-Ca(v)3.2Cter fusion protein. C, Co-immunoprecipitation of SNAP-25 with CD4-EGFP-Ca(v)3.2Cter. |
Role of Glycosylation in T-type Calcium Channel FunctionThe cellular and physiological functions supported by ion channels are not only determine by the intrinsic functional properties of the channels embedded in the plasma membrane but also by the number of channels that are expressed at the cell surface, their dynamic (stability) and more importantly by their specific expression in subcellular loci. Protein glycosylation is rapidely emerging as a fundamental post-translational mechanism controling those apsects, and defects in ion channel glycosylation give rise to numerous human disorders. Alteration of T-type channel expression during diabetes has been documented at the synaptic endings of primary nociceptive neurons, and recent work from our lab and others have shown that T-type Ca2+ channel function and expression at the plasma membrane is potently regulated by N-linked glycosylation, and depends on extracellular glucose concentration (as it is relevant for diabetes). We are exploring the role of glycosylation in the sorting and trafficking of T-type channels in nociceptive neurons to better understand their contribution in painfull diabetic neuropathy, and possibly in other diabetes-related neuronal disorders. |
|
|
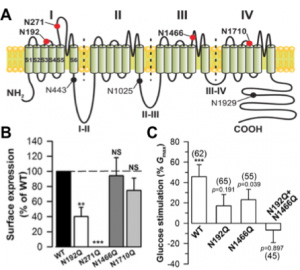
Illustrative citation: N-linked glycosylation and external glucose control T-type channel expression. A, Schematic representation of the location of potential N-linked glycosylation sites in the scheme of Ca(v)3.2 channel structure. B, Normalized surface expression of recombinant glycosylated-deficient Ca(v)3.2 channels (N192Q, N271Q, N1466Q and N1710Q) expressed in HEK cells. C, Effect of external glucose on T-type channel activity measured in HEK cells expressing wild-type and glycosylation-deficient Ca(v)3.2 channels. Data are presented as percentage of current potentiation when rising external glucose concentration from 5 mM (physiological condition) to 25 mM (diabetic-like condition). |
|
Electrophysiological Aspects of Congenital ChannelopathiesIn collaboration with clinical molecular genetic labs, we are characterizing new mutations of ion channels found in patients with neuronal and heart disorders to understand the cause, provide tools for genetic diagnosis, and ideally propose new therapeutic strategies. We are currently working on various mutations of KCNQ1 and HERG channels that are associated with the cardiac arrhythmia long QT syndrome, and may lead to syncope, cardiac arrest, or sudden death. |