Projekty
Assembly of retrovirusesLate in the retroviral life cycle, the immature viral particles are assembled from the polyprotein precursors, translational products of the gag, gag-pol or gag-pro, and gag-pro-pol genes, and two molecules of genomic RNA. The assembly can take place either in the cytoplasm of infected cells (so called D-type viruses, i.e. M-PMV and MMTV) or at the plasma membrane, concomitant with the budding process (so called C-type viruses, e.g. HIV). We have used both morphologic types of retroviruses for assembly process analysis. In collaboration with Prof. Ruml's laboratory at ICT Prague, we have shown that M-PMV Gag polyprotein can assemble into procapsids in prokaryotic cells and in in vitro system. Using both these systems, we have identified the minimal domain of M-PMV Gag ( ProCANC), necessary for the assembly of immature particles. In vitro assembled particles formed from the purified recombinant ProCANC were analyzed for molecular organization using electron microscopy. The same in vitro system employing both, MPMV and HIV truncated CANC and ProCANC domains, was used for the elucidating of the role of nucleic acids during assembly of immature particle. Current research is focused on searching (testing of) compounds, efficiently inhibiting the in vitro assembly of HIV CA and CANC proteins and in cooperation with foreign laboratories on evaluating their influence on the virus assembly in infected cells. We also investigate the architecture of M-PMV immature capsids and contribution of nucleic acids to the assembly of M-PMV. Studies on identification of cellular proteins involved in intracellular transport of M-PMV Gag polyproteins at the place of assembly are underway. In collaboration with NMR laboratory at ICT Prague, we implement in our research a structural analysis of viral proteins participating in assembly process. We also perform in vitro and in vivo experiments enabling to define an internal scaffold domain which permits intracytoplasmic assembly in Mouse mammary tumor virus. |

|
Maturation of retrovirusesThe retroviral proteins are synthesized as Gag polyprotein precursors, which are processed by virus encoded protease (PR) during or shortly after budding. This proteolytic cleavage triggers the particle re-arrangement into mature, fully infectious particles. Our main goal is to investigate a mechanism of activation of PR, i.e. the initiation of cleavage of PR domain from polyprotein precursors and formation of the active dimeric form of protease. Since assembly of immature capsids and maturation are in Mason-Pfizer monkey virus two temporary and spatially separated processes, we use M-PMV as a model for these studies. The protease of M-PMV contains unique C-terminal extension sequences, containing a glycine-rich motif (G-patch), which is removed in vitro as well as in vivo by autoproteolytic processing to yield truncated active forms of PR. The NMR structural analysis and biochemical experiments confirmed that this domain is not necessary for formation of fully folded active protease. To investigate the role of the G-patch domain in the virus replication, we use different approaches including yeast two hybrid system for searching the cellular interacting proteins, immunofluorescent microscopy for analysis of G-patch trafficking, and mutation of functionally important sequence in M-PMV protease domain followed by a detection of virus processing, infectivity, and morphology. |
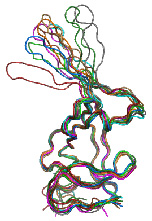
|
Functional analysis of key enzymes of Mycobacterium tuberculosisThis research contributes to the Collaborative project of the 7th framework programme Health: Systems Biology of Mycobacterium tuberculosis (SysteMtb) by the biochemical characterization of poorly characterized enzymes and by kinetic characterization of selected enzymes. |
|
Role of secreted aspartic proteases (Saps) of different Candida spp. in metabolism of pathogenic yeasts and host-pathogen interactionsYeast is a model eukaryotic organism. Although this is true mainly for Saccharomyces cerevisiae, the genomic and post-genomic era enables to compare gene and protein sequences and many more features of various yeast species. This facilitates the exploration and understanding of eukaryotic systems in general. Our group works mostly with the pathogenic yeast of the genus Candida. These opportunistic pathogens cause no harm to healthy individuals. However, they represent a serious threat to immunocompromised patients. The secretion of proteases is one of the virulence factors of pathogenic Candida spp. Together with lipases and phospholipases they facilitate the penetration of host surfaces by Candida. Therefore the specific inhibitors of Saps are considered as possible antimycotic drugs. Our current work is focused on more detailed knowledge of the structure of Saps of C. albicans, C. tropicalis, and C. parapsilosis, mapping their substrate specificities, saps gene regulation, analysis of intracellular Sap precursors trafficking, activation, and secretion of mature proteases. |
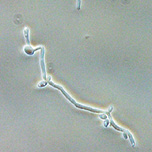
|
Functional analysis of insect desaturasesDesaturases participate in pheromone production in diverse insect species. Desaturase introduces a double bond into the aliphatic chain of the fatty acids with various specificity. The pheromone blend produced by our model organism Manduca sexta is unusually complex and contains mono-, di- and trienes. To investigate the mechanism of pheromone synthesis and a role of different types of desaturases in formation of the pheromones, we construct cDNA libraries from mRNA isolated from pheromone gland and fatty bodies and construct genes encoding the desaturases. Together with a gene analysis, we perform a functional expression of insect desaturases in Saccharomyces cerevisiae followed by GC-MS analysis of fatty acids methyl esters and their derivatized products, which helps us to identify type of isolated desaturases. This project is investigated in a close cooperation with Dr. Aleš Svatoš from Max Planck Institute for Chemical Ecology in Jena. We also cooperate with the group of Dr. Irena Valterová at IOCB on identification of the key enzymes involved in biosynthesis of pheromones of Bombus lucorum and Bombus terrestris males. |

|