Thermoelectric effect is an ability of a given material to produce voltage when temperature gradient is present, thus converting thermal energy to electric energy. Thermoelectric power generation technology is expected to improve the total efficiency of energy utilization and to suppress the consumption of fossil fuels.
Heat emitted from various sources, e.g. solar, geothermal, but first of all the waste heat from industrial processes, automobiles, etc…, can be directly converted into clean electricity by a thermoelectric (TE) device. Nowadays, the waste heat represents approximately 60% of all energy consumed. TE device has an important advantage of being simple solid assembly of a few components without any moving parts. It is compact and feasible for any kind of scaling (from miniature to large scale devices). One of the most promising applications is e.g. in automobiles, where heat lost in the engine coolant or exhaust gas can be converted into electrical energy. Actual applications include e.g. power generation in oil and gas pipelines or for deep-space probes via radioisotope thermoelectric generators. In addition, TE device can also work reversibly as a heat pump for refrigeration.
The application range is presently limited by low efficiency of the thermoelectric devices. The energy conversion capability of a TE device is determined by the Carnot efficiency and, importantly, by the dimensionless figure of merit (ZT) of the TE material:
ZT = T (S2 · σ) / λ ,
where S is the thermoelectric power or Seebeck coefficient, σ is the electrical conductivity, λ is the thermal conductivity and T is the absolute temperature.
In order to increase the parameter ZT and realize an efficient thermoelectric energy conversion, the following three physical properties are required for thermoelectric materials: (1) low thermal conductivity (λ = λL + λE, where λL and λE are the lattice and electronic contributions, respectively), which is necessary to introduce a large temperature difference into both ends of the material, (2) high electrical conductivity (σ), which is required to reduce the internal resistance of the material, and (3) large thermoelectric power (S), which is needed to obtain a high voltage.

Fig.1. The thermoelectric generator is constructed as a battery composed of suitable pairs of n and p-type materials forming so called unicouple - see the detail. The heat from hot source flows from the junction to terminal contacts, inducing a temperature gradient in the n-type and p-type "legs" placed thermally in parallel and electrically in series. The generated thermoelectric voltage is of opposite sign in the respective legs and is, therefore, additive in their serial electrical connection. The construction of the thermoelectric battery is, in addition to the availability of high quality thermoelectrically matched legs, conditioned by properly made and stable electrical and thermal contacts. Picture adopted from Ref. [1].
As evidenced from the Fig.1., both p-type (S>0) and n-type (S<0) thermoelectric materials are required to fabricate a TE module. So far, bulk materials based on Bi2Te3, PbTe and skutterudites possessing the highest ZT around 1.5 - 1.8, have been utilized to fabricate TE modules. However, these state of art thermoelectric materials are composed of in principle toxic, naturally rare, and heavy elements which melt or oxidize at high temperatures in air. Thus, these conventional TE materials cannot be employed for extensive applications for high temperature waste heat recovery in air atmosphere. To overcome these problems, novel TE materials with high ZT composed of nontoxic, naturally abundant, light, and cheap elements should be developed. Oxide materials meeting these requirements are highly promising candidates from this point of view.
In general, thermoelectric oxides can be divided into 2 classes:
(i) materials at the border between metal and insulator ground state, often with strong electron-electron correlations of electrons at Fermi energy. Here the energy gap is rather of the Mott-Hubbard type (i.e. caused by the electron-electron correlation) and the doping/self doping mechanism results in mixed valence. The itinerancy of conducting electrons is achieved due to suppressed electron correlations, the mechanism substantially different than that known for 'classic' semiconductors. The layered cobaltites NaxCoO2 and Ca3Co4O9 or n-type electrically conducting CaMnO3 like (doped Ca1-x2+Lnx3+Mn1-y-z4+Mey3+TMz5+O3; Ln=Lanthanide, TM=transition metal) belong to this type of materials.
The second class (ii) of thermoelectric oxides is represented by wide gap semiconductors with low or moderate doping, examples are Sr1-x2+Lax3+Ti1-y4+Tiy3+O3 and Zn1-x2+TMx3+O. In that case the gap is of the charge transfer type, contrary to the previous case, and the doping mechanism (insinuated in the chemical formulas) is similar to that known in 'classic' semiconductors.
The research of Laboratory of oxide materials in the field of thermoelectricity comprises both the routes to optimise the thermoelectric oxide based materials aimed to high temperature applications and possibilities of the thermoelectric waste heat recovery from the exhaust gas of automobile heat engine. Due to the lack of commercial thermoelectric characterisation tools which are either unavailable or not trustworthy we focused essentially on measurement automation and substantial thermoelectric metrology refinement in the evaluation of thermal, thermoelectric and electric power characteristics of newly developed materials and thermoelectric modules, Ref. [2]. This was achieved mainly due to more accurate thermometry (error determining the temperature of ~0.05 K) and the stabilization of the cooling bath. Detail of the measuring apparatus including thermography sensing surface temperature is shown in Fig.2.
Our fundamental research is mainly devoted to layered cobaltites NaxCoO2 and Ca3Co4O9 (see e.g. Refs. [3-6]) and, as regards non-oxide materials, to Chevrel's cluster compounds and Skutterudites (see e.g. Refs. [7-9]).In addition, novel layered cobaltates Ln0.30CoO2 (Ln=La,Pr,Nd,...) were prepared from NaxCoO2 precursor by a solid-state ionic exchange, see Refs. [10-12]. The compound consists of hexagonal sheets of edge-sharing CoO6 octahedra, alternating with planes of Ln3+ cations with the trigonal prismatic coordination, where Ln3+ ions occupy only one third of available sites and forms a 2-dimensional superstructure, see Fig.3. The system displays promising thermoelectric properties and, in comparison with the parent system NaxCoO2, it allows study of the mutual influence of magnetic ion Ln3+ and the CoO2 layer.
 |
 |
| Fig.2. Automated characterization equipment for testing of thermoelectric modules and materials. | Fig.3. The structure of Ln0.30CoO2. Ln(green), vacant sites(white), octahedra CoO6(magenta and blue). |
 |
 |
 |
| Fig.4. Structure of p-type NaxCoO2. Octahedra CoO6(red), Na(magenta). | Fig.5. Structure of p-type Ca3Co4O9. Octahedra CoO6(red), Co(red), Ca(yellow), O(blue). | Fig.6. Structure of n-type CaMnO3. Octahedra MnO6(green), Ca(blue). |
 |
 |
|
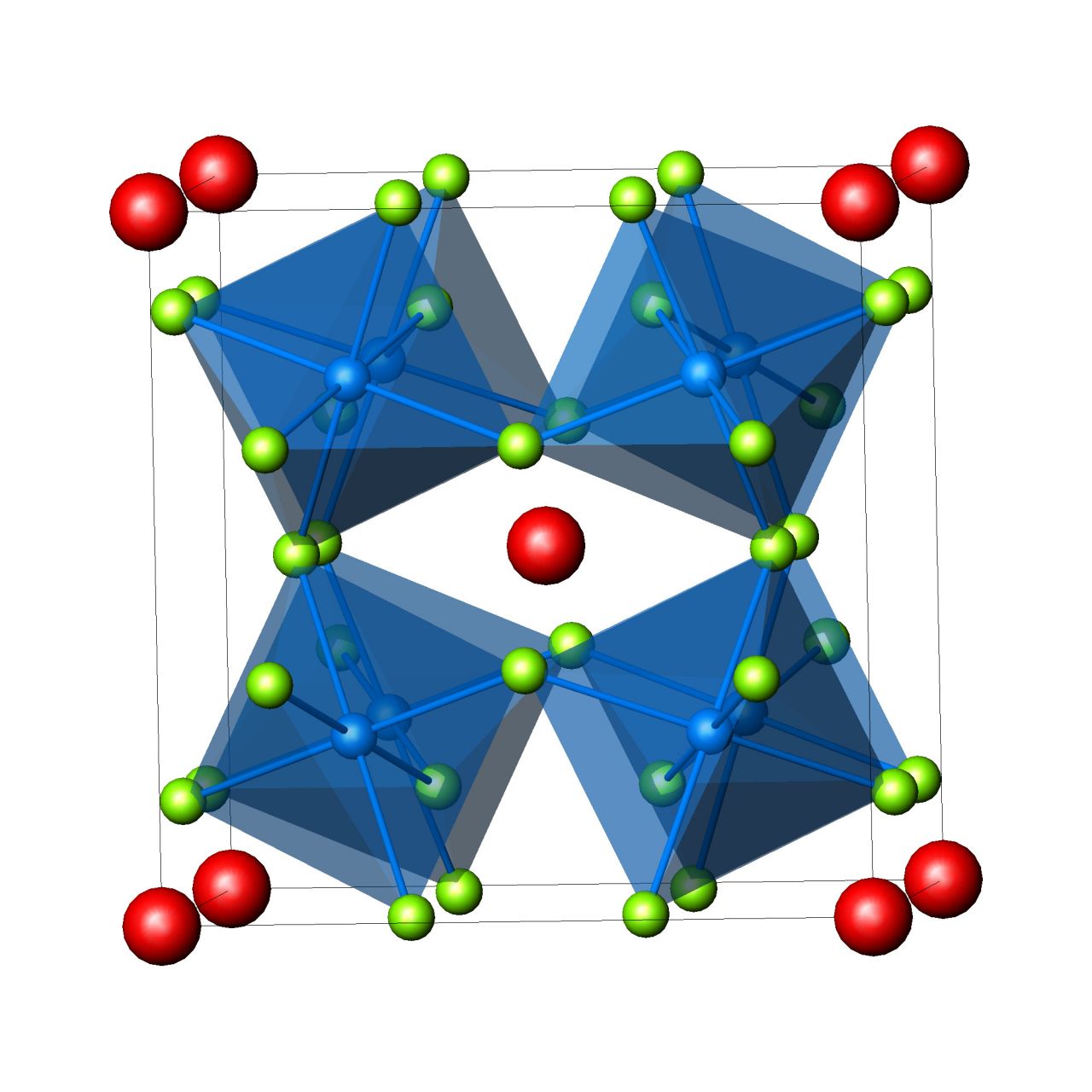
| Fig.7. Structure of Cu2Se. Se(yellow), Cu(red). | Fig.8. Structure of the Chevrel's phase CuxMo6Se8. Mo6 clusters(blue), Se(yellow), Cu(red). | Fig.9. Structure of partially filled skutterudites with general formula AyM4X12. MX6 octahedra(blue), X(green), A(red). |
 |
 |
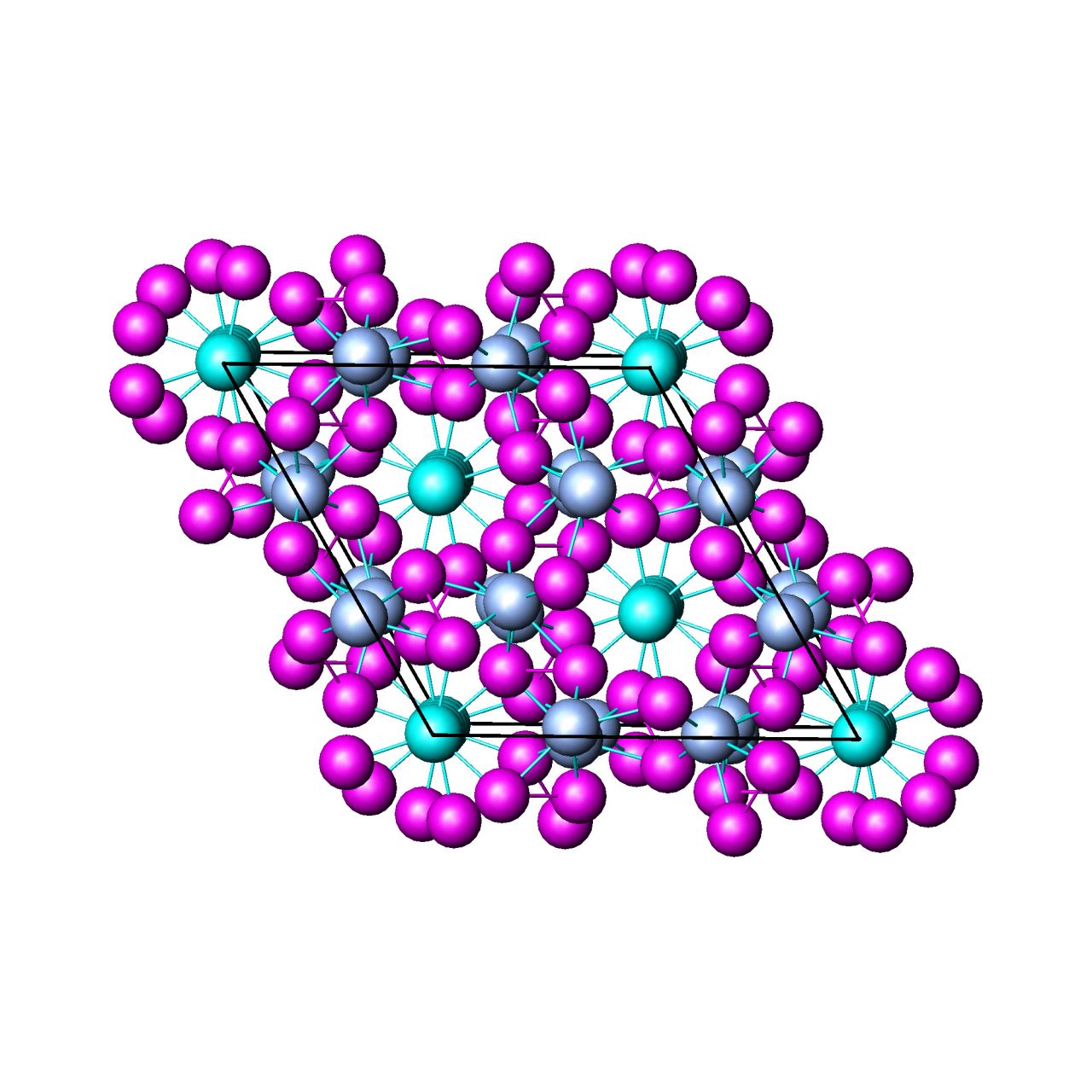 |
| Fig.10. Structure of the type-I clathrate Sr8Ga16Ge30. Clusters of (GaGe)20(magenta) and (GaGe)24(red) are filled by Sr atoms. | Fig.11. Tetragonal structure of Zintl's phase Yb14MnSb11, view along c-axis. Tetrahedra MnSb4(blue), Yb(magenta), Sb(green). | Fig.12. Structure of Zn4Sb3. Zn(magenta), Sb(light blue), mixed Sb/Zn(dark blue). |
Copyright © 2008-2014, Fyzikální ústav AV ČR, v. v. i.