Projekty
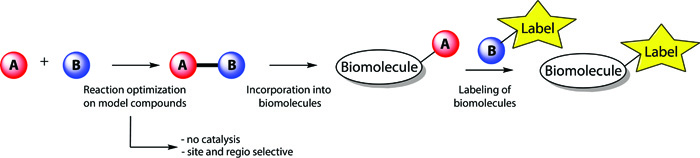
New Bioconjugation ReactionsBioorthogonal chemical reactions emerged as a powerful tool of modern organic chemistry. They substantially extended our ability to precisely manipulate and label biomolecules. Although great progress was done in this direction, there is still a strong desire for new methods that address the shortcomings of the known technologies. In this project we systematically study various chemical transformations as potentially novel bioconjugation reactions. We focus on organic reactions that proceed in aqueous medium, are chemoselective and orthogonal to other functional groups. Measurements of the rate constants by HPLC or UV/Vis enable to identify most reactive compounds, which are further optimized regarding their stability and selectivity under physiological conditions. Synthesis of precursors that enable to incorporate the hit compounds into biomolecules is then performed and the introduced artificial functional group is used to decorate biomolecules with useful tags and markers (e.g. PEG, fluorophore or biotin).
 |
|
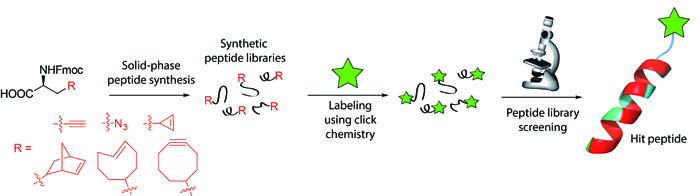
Synthesis of Peptide Libraries Containing Unnatural Amino AcidsArtificial amino acids containing different clickable functional groups are designed and synthesized. Optimization of the conditions used in solid-phase peptide synthesis is performed to achieve efficient incorporation of these nonstandard amino acids into peptide libraries. The introduced clickable moiety is used to attach small molecule drugs or reporter groups to these peptides. The combinatorial peptide libraries are screened to find sequences with high binding affinity to therapeutically interesting enzymes. These small molecule-peptide conjugates are then used as new class of drug delivery systems or applied for diagnostic purposes. This concept enables to reach unprecedented selectivity to enzymes and proteins involved in pathological processes.
 |
|
Selective Inhibitors of GlycosidasesCarbohydrate processing enzymes play crucial role in all living systems. Although these proteins act on various substrates, they are characterized by remarkable selectivity. Inhibition of enzymes acting on carbohydrates represents a new promising strategy in the treatment of various diseases. This project combines the biological activity of known glycosidase inhibitors with combinatorial peptide synthesis to select peptide-small molecule inhibitors with improved properties and binding affinities. Using rational design, analogs of glycosidase inhibitors are synthesized and attached to peptide libraries using biocompatible chemistry. These bioconjugates are screened for specific binding to particular glycosidases. Besides therapeutic applications, these constructs are used for dissecting the role and function of individual glycosidase family members in living systems. |
|