Projekty
RNAse LRNase L is an interferon-induced ribonuclease which, upon activation, destroys all RNA within the cell (both cellular and viral). RNase L is present in very minute quantities during the normal cell cycle. When interferon binds to cell receptors, it activates transcription of around 300 genes to bring about the antiviral state. Among these genes is RNase L, which is initially produced in inactive form.
Activation occurs by the following:
|
|
CpG Motif Containing Oligonucleotides and Their Interaction with TLR9TLR 9 (toll-like receptor) recognizes unmethylated CpG sites on DNA molecules. CpG sites are extremely rare (~1%) on vertebrate genomes but prevalent on bacterial and viral DNA. TLR9 is expressed by numerous cells of the immune system such as dendritic cells, B lymphocytes and natural killer (NK) cells. TLR9 is expressed intracellularly, within the endosomal compartments and functions to alert the immune system of viral and bacterial infections by binding to DNA rich in CpG motifs. TLR9 signals leads to activation of the cells initiating pro-inflammatory reactions that result in the production of cytokines such as type-I inteferon and IL-12. There are new immunomodulatory treatments undergoing testing which involve the administration of artificial DNA oligonucleotides containing the CpG motif. CpG DNA has applications in treating allergies such as asthma, immunostimulation against cancer, immunostimulation against pathogens, and as adjuvants in vaccines. This project deals with design and synthesis of modified oligonucleotides containing CpG motifs. 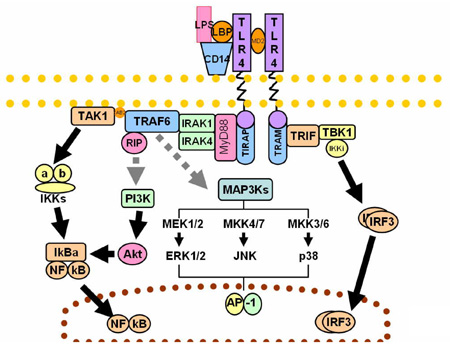 |
|
5'-NucleotidasesThe monophosphate 5'-nucleotidases, including 5'(3')-deoxyribonucleotidase,form a family of enzymes that catalyze the dephosphorylation of nucleoside monophosphatesand regulate cellular nucleotide and nucleoside pools levels. The ribonucleotides and deoxyribonucleotides could be synthesized de novo from low-molecularweight precursors or by salvage from nucleosides or nucleobases coming from catabolism of nucleic acids. In the salvage pathway, ribonucleotides and deoxyribonucleotides are phosphorylated by nucleoside and nucleotide kinases to maintain sufficient pools of dNTP's and NTP's for synthesis of DNA and RNA. The phosphorylation by cellular nucleoside kinases is opposed by 5'-nucleotidases that dephosphorylate ribo- and deoxyribonucleoside monophosphates. Besides their role in the regulation of physiological dNTP pools, substrate cycles between ribonucleotidases and kinases may affect the therapeutic action of pyrimidine nucleoside analogs used as anticancer and antiviral agents. Such compounds require the nucleoside kinases activity for phosphorylation to their active forms. Clinical and in vitro studies suppose that an increase in nucleotidase activity can interfere with nucleoside analogue activation resulting in drug resistance. |
Nucleoside PhosphorylasesPurine Nucleoside Phosphorylase (also known as PNPase) is an enzyme involved in purine metabolism. PNP metabolizes adenosine into adenine, inosine into hypoxanthine, and guanosine into guanine, in each case creating ribose phosphate. NP encodes the enzyme purine nucleoside phosphorylase that together with adenosine deaminase (ADA) serves a key role in purine catabolism, referred to as the salvage pathway. Mutations in either enzyme result in a severe combined immunodeficiency (SCID). Uridine phosphorylase is an enzyme that catalyzes the chemical reaction: {uridine + phosphate → uracil + α-D-ribose 1-phosphate} Thus, the two substrates of this enzyme are uridine and phosphate, whereas its two products are uracil and α-D-ribose 1-phosphate. This enzyme belongs to the family of glycosyltransferases, specifically the pentosyltransferases. This enzyme participates in pyrimidine metabolism. |
|
|
Thymidine Phosphorylase is an enzyme that catalyzes the chemical reaction: {thymidine + phosphate → thymine + 2-deoxy-α-D-ribose 1-phosphate} Thus, the two substrates of this enzyme are thymidine and phosphate, whereas its two products are thymine and 2-deoxy-α-D-ribose 1-phosphate. This enzyme belongs to the family of glycosyltransferases, specifically the pentosyltransferases. This enzyme participates in 3 metabolic pathways: purine metabolism, pyrimidine metabolism, and bladder cancer. Much attention was devoted to the study of potential inhibition of thymidine phosphorylase (TP), an enzyme playing important role in angiogenesis. We introduced a high-throughput absorbance test utilizing 2'-deoxy-5-nitro-uridine as a TP substrate, which allows for monitoring the real-time activity of the enzyme. We tested more than 80 nucleoside-phosphonic acids as possible bi-substrate inhibitors of TP from various sources. Certain interesting relationships were found, and they are now subject to a further assessment process. |
|
Phosphonate Analogues of Nucleotides - cytostatic, virostatic, antimalarial propertiesWe do synthesis of various analogues of nucleotides. We are mainly focused in modification of sugar and phosphate moiety. Prepared compound are tested against wide spectrum of pathogens by our collaborators. |
|