DNA strand breaks, RNA processing, neurodegenerative diseases
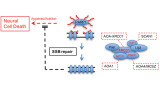
DNA single-strand breaks (SSBs) are amongst the most frequent DNA lesions arising in cells and can threaten genetic integrity and cell survival. One of the proteins important for rapid repair of SSBs is XRCC1. XRCC1 is a molecular scaffold protein that interacts with multiple DNA repair enzymes (e.g. PARP1, PNKP, Polβ, APTX, Lig3) thus promoting their stability and/or function. Interestingly, we and others have shown that SSB repair defects are associated with hereditary neurodegeneration in humans, as illustrated by the genetic diseases ataxia oculomotor apraxia type XRCC1 (AOA-XRCC1; mutated in XRCC1), ataxia oculomotor apraxia-1 (AOA1; mutated in APTX), spinocerebellar ataxia with axonal neuropathy-1 (SCAN1; mutated in TDP1), and microcephaly with early onset seizures/ataxia oculomotor apraxia-1 (MCSZ/AOA4; both mutated in PNKP). However, the mechanisms by which SSBs trigger neurodegeneration, and to what extent SSBs contribute to other neurodegenerative diseases such as the dominant cerebellar ataxias, Huntington’s and Parkinson’s diseases, and dementias such as Alzheimer’s are unknown.
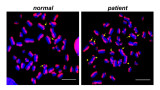
Recently, we have defined the cellular and pathological consequences of XRCC1 loss in human and mouse brain. XRCC1-mutant patient cells exhibited elevated levels of ADP-ribose, a phenotype that was recapitulated in a related syndrome caused by mutations in the XRCC1 partner protein PNKP and implicating hyper-activation of the SSB sensor protein PARP1 as a putative source of neurotoxicity. Indeed, remarkably, genetic deletion of Parp1 rescued normal cerebellar ADP-ribose levels and prevented the loss of cerebellar neurons and ataxia in Xrcc1-defective mice. We thus established the importance of XRCC1 protein complexes for normal neurological function and identify SSB sensor protein PARP1 as a therapeutic target in DNA strand break repair-defective disease.
We now propose that the impact of SSBs on neurodegeneration may extend beyond rare SSB repair-defective diseases to include more common motor neurone diseases and genetically dominant spinocerebellar ataxias, as well as Alzheimer’s, Huntington’s and Parkinson’s diseases. Ultimately, we suggest that SSBs might even be an etiological factor in normal human ageing. Finally, we propose that SSBs induce neurodegeneration by triggering over-activation of the SSB sensor protein, PARP1; thereby identifying inhibitors of this protein (which are currently licensed for treatment of cancer) as a possible therapeutic approach for the treatment of neurodegenerative disease. We aim to identify additional novel regulators of the cellular response to SSBs that might be linked with neurodegenerative disease, and to exploit our findings therapeutically.
Outstanding young scientists interested in postdoctoral positions in the Laboratory of Genome Dynamics should contact Keith directly at k.w.caldecott@sussex.ac.uk.
See also www.sussex.ac.uk/lifesci/caldecottlab.
Last change: September 22, 2017