2016
From helical to planar chirality by on-surface chemistry
Scientists from the Institute of Organic Chemistry and Biochemistry CAS and Institute of Physics CAS succeeded in imaging chemical transformations of single molecules at the silver surface to demonstrate a chirality transfer in these reactions. Employing the newest methods of scanning probe microscopy they achieved an ultimate resolution in visualising chemical bonds between individual atoms to determine the exact molecular structure along with its chirality.
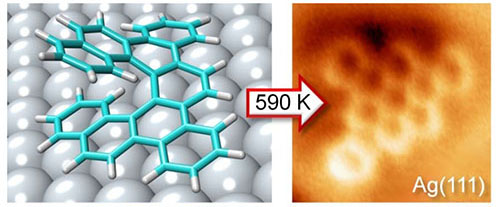
|
Transformation of individual molecules and their chirality on the silver surface
The picture shows a transformation of the optically pure 3D helical molecule (an optimised model) into planar 2D aromatics adsorbed on the silver surface (an experimental AFM image) that exhibits planar chirality. |
Stetsovych O., Švec M., Vacek J., Vacek Chocholoušová J., Jančařík A., Rybáček J., Kosmider K., Stará I.G., Jelínek P., Starý I.:
From helical to planar chirality by on-surface chemistry.
Nature Chemistry 79: 213–218 (2017)
In cooperation with: Institute of Physics of the Czech Academy of Sciences
Contacts: Dr. Ivo Starý [ivo.starý@uochb.cas.cz].
A non-exploding alkali metal drop on water: From blue solvated electrons to bursting molten hydroxide
Due to their reactivity electrons in water have only a very short lifetime of the order of µs. At the time of their discovery using flash photolysis it was predicted they could never be seen by a naked eye. This is not true. By careful manipulation researchers managed to keep the vigorous reaction of the sodium/potassium alloy just below explosion. For more than a 1 second electrons were massively released into water with their blue color clearly visible by a naked eye and spectrum recorded by optical spectrometer.
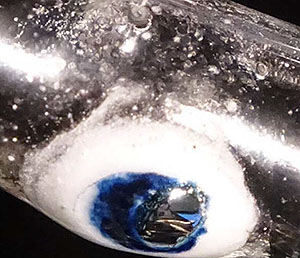
|
Blue electrons in water
A snapshot demonstrating the vigorous release of electrons (blue color) from a drop of a sodium/potassium alloy (metal "mirror" at the glass wall) into water forming gaseous hydrogen and hydroxide (white color). |
Mason P.E., Buttersack T., Bauerecker S., Jungwirth P.:
A Non-Exploding Alkali Metal Drop on Water: From Blue Solvated Electrons to Bursting Molten Hydroxide.
Angewandte Chemie International Edition 55: 13019-13022 (2017).
Contacts: Prof. Pavel Jungwirth [pavel.jungwirth@uochb.cas.cz].
Chemically modified insulins with improved receptor binding specificity
Insulin is a key hormone of human metabolism with a major therapeutic importance for a treatment of diabetes. The interaction of insulin with the metabolic type B of the insulin receptor is necessary for optimum control of glycaemia. In the contrary, the type A of the receptor mediates mainly growth effects of insulin. One of new derivatives was markedly more active than natural insulin at binding to the receptor, and moreover, it had a significant preference for the type B of the receptor.
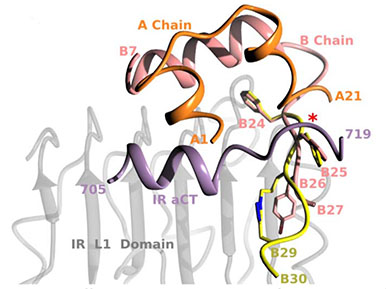
|
Highly active crosslinked insulin derivative sitting on the insulin receptor
Insulin A chain is shown in coral, insulin B chain is in pink and the crosslinked chain is in yellow with triazole nitrogen atoms shown in blue. Insulin receptor is represented by L1 domain (in grey) and aCT segment (in violet). |
Viková J., Collinsová M., Kletvíková E., Buděšínský M., Kaplan V., Žáková L., Veverka V., Hexnerová R., Avinó R.J.T., Straková J., Selicharová I., Vaněk V., Wright D.W.,
Watson C.J., Turkenburg J.P., Brzozowski A.M., Jiráček J.:
Rational steering of insulin binding specificity by intra-chain chemical crosslinking.
Scientific Reports 6: 19431 (2016).
In cooperation with: York Structural Biology Laboratory, Department of Chemistry, University of York, Heslington, York YO10 5DD, United Kingdom
Contacts: Dr. Jiří Jiráček [jiri.jiracek@uochb.cas.cz].