A bacterial rhomboid protein exhibiting both protease and pseudoprotease activity
3 February 2020
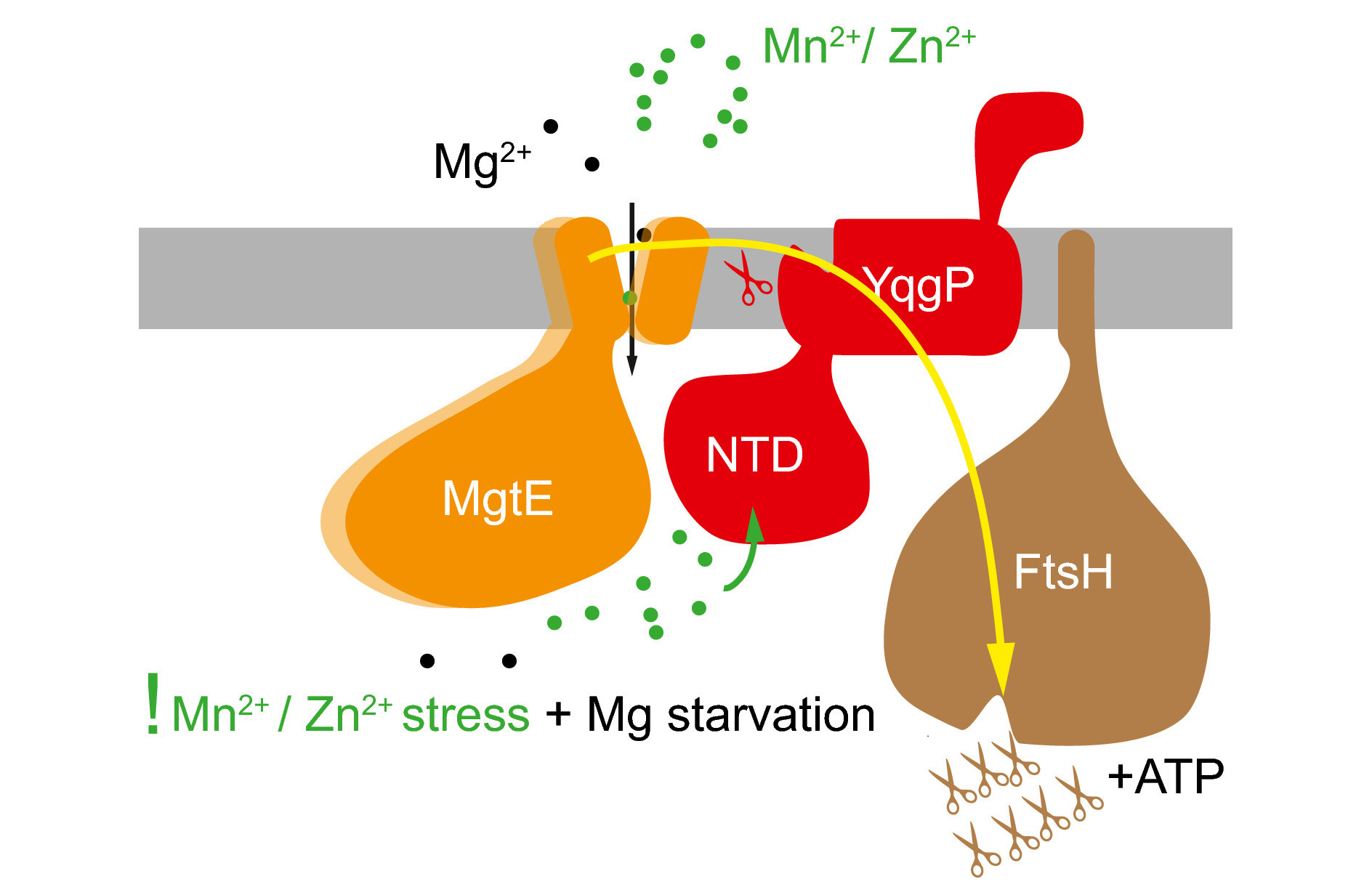
As a soil-dwelling bacterium, Bacillus subtilis needs tight control over its mineral resources. Therefore, it employs multiple control mechanisms that react to the conditions and regulate the uptake of the metal ions from its surroundings and their export from the cytosol. One such mechanism was discovered, investigated and described by a collaboration of scientists led by Kvido Stříšovský from IOCB Prague and Thierry Doan from CNRS / Aix-Marseille University and published in The EMBO Journal.
This mechanism helps control magnesium homeostasis under the conditions poor in magnesium and rich in competing ions – manganese and zinc. It is based on the YqgP rhomboid protease that, under such conditions, cleaves and inactivates a form of the major magnesium transporter of the bacterium, the MgtE, that also exhibits affinity to the competing ions and would, therefore, facilitate Mn2+ or Zn2+ toxicity instead of its physiological function.
A surprising revelation was that the YqgP enzyme behaves not only as a protease but also as a pseudoprotease activating the FtsH protease to further facilitate degradation of MgtE. This dual mode of action not only underlines the importance of fast and efficient metal ion homeostasis regulation in soil bacteria but also sheds some light into the evolution, evolutionary conservation and wide distribution of rhomboid proteases and pseudoproteases among both prokaryotes and eukaryotes. Furthermore, it provides a link between eukaryotic and prokaryotic molecular machinery specialized in the recognition and degradation of faulty transmembrane proteins.
Original paper: Began, J.; Cordier, B.; Březinová, J.; Delisle, J.; Hexnerová, R.; Srb, P.; Rampírová, P.; Kožíšek, M.; Baudet, M.; Couté, Y.; Galinier, A.; Veverka, V.; Doan, T. & Strisovsky, K. Rhomboid Intramembrane Protease YqgP Licenses Bacterial Membrane Protein Quality Control as Adaptor of FtsH AAA Protease. The EMBO Journal 2020. doi:10.15252/embj.2019102935
Read next...