Head
prof. Mikael Kubista, PhD
prof. Mikael Kubista, PhD
Research Topics
The Laboratory of Gene Expressionis a Europe‘s leadingacademic laboratory specialized in highthroughput gene expression profiling and single-cell analysis using real- time quantitative PCR (qPCR). We participate in several basic research projects in the field of developmental biology and stem cells, several applied projects of clinical and medical relevance, in particular in cancer and neurological research, and we are involved in development of methods and applications including standardization.
Our developmental research uses the model organism Xenopus laevis. Xenopus has many advantages such as outer fertilization and outer embryonic development, indu- cible ovulation, large size of oocytes, large number of ovulated oocytes, transparent embryos and convenient conditions for breeding. Measuring temporal profiles of key developmental biomolecules (microRNAs, mRNAs and proteins) in individual blasto- meres in the earliest developmental stages (1 to 64 cells) we can monitor the forma- tion of gradients that underlie differentiation and eventually lead to the formation of body axis. We developed qPCR tomography to measure gradients even within cells and revealed that several mRNAs are polarized already in the original oocyte. The Xenopus oocyte has two differentially coloured hemispheres called animal and vegetal. The oocyte was cryo-sliced into 30-μmthin slices along the animal-vegetal axis, mRNA was isolated, cDNA prepared and quantified with qPCR. We observed three groups of maternal mRNAs, with different polarizations in the oocyte. One group of mRNAs was most abundant in the animal hemisphere. The other two groups were more abundant in the vegetal hemisphere, with the mRNAs in the third group being exclusively found in the most distal part of the vegetal hemisphere. The gradients remain throughout the initial cell divisions.
We study the role of glial cells during development and repair. Glial cells are implicated in neurodevelopment, synapse formation, neuroprotection, regenerative mechanisms after CNS injury, and the maintenance of extracellular ionic and transmitter homeostasis. Astrocytes is the main glial cell type in the brain and is involved in the maintenance of the blood-brain barrier, regulation of water and ionic homeostasis the metabolism of amino acid neurotransmitters, as well as providing energy and nutrient support to neurons.
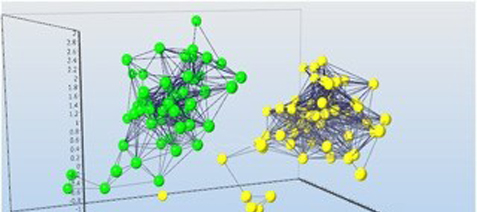
Using single cell expression profiling we found several distinct astrocytic populations to be present in the mouse cortex that seem to correlate to the extent of cell swelling during oxygen-glucose deprivation. Using the inhibitors we studied the contribution of Cl- and K+ channels, co-transporters and excitatory amino acid transporters to oxygen-glucosedeprivationinducedchangesoftheastrocyticvolume. We found two main astrocytic subpopulation that differ in expression of inwardly rectifying K+ channels (Kir4.1), K(2P) channels (TREK-1 andTWIK-1), and Cl- channels (ClC2). Using mouse model, collecting astrocytes at 10, 20, 30 and 50 days after birth and 3, 7 and 14 days after focal cerebral ischemia for profiling we found transcriptional fully mature astrocytes express mainly Eaat1, Glul, Aqp4 and Kcnj10. After a brain injury the situation rapidly changes: the astrocytes are reactive and express Eaat1, Glul, Aqp1, Aqp9, Snap25, hyperpolarization-activated cation channels Hcn1, Hcn2, Hcn3, glutamate receptors Grin2a, Gria2, Gria3, Grm5 and potassiumchannels Kcna3.
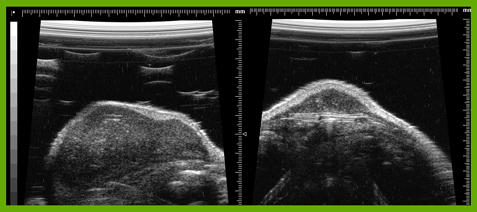
We have developed SOP for expression profiling of cancer cells extracted fromcircula- tion (blood) and fromprimary tumours. A multivariate model for the analysis of expres- sion results has been developed and is evaluated for use in therapy. The presence of the tumour cells in circulation is a very poor prognostic factor for patients, indicating metastasis, and characterization of the tumour cells by means of expression profiling can have significant value as a therapy indicator. By expression profiling we can identify cancer stem-like cells, which are more aggressive because of their ability to differenti- ate and their higher resistance to drugs. Currently, in collaboration with AdnaGen and TATAA Biocenter we are evaluating a panel of 24 markers for the profiling and charac- terization of circulating tumour cells that will be used to guide treatment.
The laboratory is also part of the European project SPIDIA (www.spidia.eu) developing methods for optimization of the pre-analytical processes of molecular diagnostics. Our contributions consist in qPCR analysis of mRNA in blood and tissue samples. Several studies report that degradation and deregulation processes take place during the pre- analytical procedures when the sample is prepared for analysis, and the measured profiles are influenced by the handling of the samples rather than by the underlying biology.Currently, noreliablebiomarkers areavailabletoidentify thechanges that occur during the pre-analytical process. We have monitored and validated a set of biomarker candidates to assess the pre-analytical variation of mRNA levels in the blood samples collected in EDTA and PAXgene tubes that would reflect degradation/deregulation.
Our laboratory also develops working cooperations with other institutes, for example Institute of Experimental Medicine. In our mutual project, we investigate functional and molecular markers of DNA repair in tumor and healthy tissues froma group of patients with sporadic forms of colorectal carcinom. An important aspect of the project is to clarify theabnormal epigenetic profile(methylation) of tumour cells intheconcomitance with their genetic constitution, which will improve our understanding of the different sporadic colorectal carcinomphenotypes.
Our developmental research uses the model organism Xenopus laevis. Xenopus has many advantages such as outer fertilization and outer embryonic development, indu- cible ovulation, large size of oocytes, large number of ovulated oocytes, transparent embryos and convenient conditions for breeding. Measuring temporal profiles of key developmental biomolecules (microRNAs, mRNAs and proteins) in individual blasto- meres in the earliest developmental stages (1 to 64 cells) we can monitor the forma- tion of gradients that underlie differentiation and eventually lead to the formation of body axis. We developed qPCR tomography to measure gradients even within cells and revealed that several mRNAs are polarized already in the original oocyte. The Xenopus oocyte has two differentially coloured hemispheres called animal and vegetal. The oocyte was cryo-sliced into 30-μmthin slices along the animal-vegetal axis, mRNA was isolated, cDNA prepared and quantified with qPCR. We observed three groups of maternal mRNAs, with different polarizations in the oocyte. One group of mRNAs was most abundant in the animal hemisphere. The other two groups were more abundant in the vegetal hemisphere, with the mRNAs in the third group being exclusively found in the most distal part of the vegetal hemisphere. The gradients remain throughout the initial cell divisions.
We study the role of glial cells during development and repair. Glial cells are implicated in neurodevelopment, synapse formation, neuroprotection, regenerative mechanisms after CNS injury, and the maintenance of extracellular ionic and transmitter homeostasis. Astrocytes is the main glial cell type in the brain and is involved in the maintenance of the blood-brain barrier, regulation of water and ionic homeostasis the metabolism of amino acid neurotransmitters, as well as providing energy and nutrient support to neurons.
Using single cell expression profiling we found several distinct astrocytic populations to be present in the mouse cortex that seem to correlate to the extent of cell swelling during oxygen-glucose deprivation. Using the inhibitors we studied the contribution of Cl- and K+ channels, co-transporters and excitatory amino acid transporters to oxygen-glucosedeprivationinducedchangesoftheastrocyticvolume. We found two main astrocytic subpopulation that differ in expression of inwardly rectifying K+ channels (Kir4.1), K(2P) channels (TREK-1 andTWIK-1), and Cl- channels (ClC2). Using mouse model, collecting astrocytes at 10, 20, 30 and 50 days after birth and 3, 7 and 14 days after focal cerebral ischemia for profiling we found transcriptional fully mature astrocytes express mainly Eaat1, Glul, Aqp4 and Kcnj10. After a brain injury the situation rapidly changes: the astrocytes are reactive and express Eaat1, Glul, Aqp1, Aqp9, Snap25, hyperpolarization-activated cation channels Hcn1, Hcn2, Hcn3, glutamate receptors Grin2a, Gria2, Gria3, Grm5 and potassiumchannels Kcna3.
We have developed SOP for expression profiling of cancer cells extracted fromcircula- tion (blood) and fromprimary tumours. A multivariate model for the analysis of expres- sion results has been developed and is evaluated for use in therapy. The presence of the tumour cells in circulation is a very poor prognostic factor for patients, indicating metastasis, and characterization of the tumour cells by means of expression profiling can have significant value as a therapy indicator. By expression profiling we can identify cancer stem-like cells, which are more aggressive because of their ability to differenti- ate and their higher resistance to drugs. Currently, in collaboration with AdnaGen and TATAA Biocenter we are evaluating a panel of 24 markers for the profiling and charac- terization of circulating tumour cells that will be used to guide treatment.
The laboratory is also part of the European project SPIDIA (www.spidia.eu) developing methods for optimization of the pre-analytical processes of molecular diagnostics. Our contributions consist in qPCR analysis of mRNA in blood and tissue samples. Several studies report that degradation and deregulation processes take place during the pre- analytical procedures when the sample is prepared for analysis, and the measured profiles are influenced by the handling of the samples rather than by the underlying biology.Currently, noreliablebiomarkers areavailabletoidentify thechanges that occur during the pre-analytical process. We have monitored and validated a set of biomarker candidates to assess the pre-analytical variation of mRNA levels in the blood samples collected in EDTA and PAXgene tubes that would reflect degradation/deregulation.
Our laboratory also develops working cooperations with other institutes, for example Institute of Experimental Medicine. In our mutual project, we investigate functional and molecular markers of DNA repair in tumor and healthy tissues froma group of patients with sporadic forms of colorectal carcinom. An important aspect of the project is to clarify theabnormal epigenetic profile(methylation) of tumour cells intheconcomitance with their genetic constitution, which will improve our understanding of the different sporadic colorectal carcinomphenotypes.
Selected Papers
Sindelka, R., Jonak, J., Hands, R., Bustin, A., Kubista, M. Intracellular expression profiles measured by real-time PCR tomography in the Xenopus laevis oocyte. Nucleic Acids Research, 36: 387-392, 2008. ISSN 0305-1048.
Bustin, A., Benes, V., Garson, J. A., Hellemans, J., Huggett, J., Kubista, M., Mueller, R., Nolan, T., Pfaffl, M.V., Shipley, G. L., Vandesompele, J., Wittver, C.T.The MIQE Guide- lines: Minimum Information for Publication of Quantitative Real-Time PCR Experiment Clinical Chemistry, 55: 611-622, 2009. ISSN 0009-9147.
Tichopad, A., Kitchen, R., Riedmaier, I., Becker, Ch., Ståhlberg, A., Kubista, M. Design and Optimization of Reverse-Transcription Quantitative PCR Experiment Clinical Che- mistry, 55:1816-1823, 2009. ISSN 0009-9147.
Laurell, H., Iacovoni, J., Abot, A., Svec, D., Maoret, J.-J., Arnal, J.-F., Kubista, M. Correction of RT-qPCRdata for genomic DNA-derived signals with ValidPrime. Nucleic Acids Research, 40: 1-10, 2012. ISSN 0305-1048.
Slyskova, J., Korenkova, V., Collins, A.R., Prochazka, P., Vodickova, L., Svec, J., Lipska, L., Levy, M., Schneiderova, M., Liska, V., Holubec, L., Kumar, R., Soucek, P., Naccarati, A., Vodicka, P. Functional, Genetic, and Epigenetic Aspects of Base and Nucleotide Excision Repair in Colorectal Carcinoma. Clinical Cancer Research 2012 Oct 19. ISSN 1078-0432.
Bustin, A., Benes, V., Garson, J. A., Hellemans, J., Huggett, J., Kubista, M., Mueller, R., Nolan, T., Pfaffl, M.V., Shipley, G. L., Vandesompele, J., Wittver, C.T.The MIQE Guide- lines: Minimum Information for Publication of Quantitative Real-Time PCR Experiment Clinical Chemistry, 55: 611-622, 2009. ISSN 0009-9147.
Tichopad, A., Kitchen, R., Riedmaier, I., Becker, Ch., Ståhlberg, A., Kubista, M. Design and Optimization of Reverse-Transcription Quantitative PCR Experiment Clinical Che- mistry, 55:1816-1823, 2009. ISSN 0009-9147.
Laurell, H., Iacovoni, J., Abot, A., Svec, D., Maoret, J.-J., Arnal, J.-F., Kubista, M. Correction of RT-qPCRdata for genomic DNA-derived signals with ValidPrime. Nucleic Acids Research, 40: 1-10, 2012. ISSN 0305-1048.
Slyskova, J., Korenkova, V., Collins, A.R., Prochazka, P., Vodickova, L., Svec, J., Lipska, L., Levy, M., Schneiderova, M., Liska, V., Holubec, L., Kumar, R., Soucek, P., Naccarati, A., Vodicka, P. Functional, Genetic, and Epigenetic Aspects of Base and Nucleotide Excision Repair in Colorectal Carcinoma. Clinical Cancer Research 2012 Oct 19. ISSN 1078-0432.
Detail webpage of Department of Gene Expression
The Institute of Biotechnology is member of: BIOCEV, CzechBIO, The Technology Centre ASCR, BIOCEV z.s.p.o.