A joint work by Czech, Spanish and Swiss scientists published by Nature Nanotechnology this week, introduces a new approach to the development of non-metallic conductors which could be used in solar energy, optical technologies or nanoelectronics.
An international team of scientists including researchers from the Regional Centre of Advanced Technologies and Materials (RCPTM) of the Faculty of Natural Sciences of the Palacky University, Olomouc, and from the Institute of Physics (FZU), Prague, has proposed and experimentally verified the possibility of preparing single-dimensional carbon-based conductive polymers. As carbon is one of the most accessible elements, the new polymer conductors have shown potentially lower production costs than normal metallic conductors, along with greater stability and the opportunity to control the properties of the material. The joint work of Czech, Spanish and Swiss scientists, published this week in the journal Nature Nanotechnology1 this week, introduces a new approach to designing non-metallic conductors, which could be used in solar energy applications, optical technologies or nanoelectronics. The significance of this work is evidenced by the fact that the article was given a special commentary by the editors of Nature Nanotechnology.
"The advantage of the new polymers is the possibility of controlling their electronic and optical properties along with expected higher stability compared to current conductive polymers. The possibility of constructing stable carbon conductive polymers paves the way for miniaturizing and enhancing the performance of a number of electronic components," says Pavel Jelínek, who leads the Czech team.
Metallic conductors, which are currently an integral part of most commercial electrical and electronic devices, pass an electrical current by means of free electrons in their structures. In most cases, organic carbon and hydrogen-based molecules do not contain free electrons and therefore act as insulators. However, organic conductors, so-called conductive polymers, are known to carry electric current thanks to enrichment by other elements. These elements supply or extract electrons from the structure of carbon polymers, creating the essential free electrical charge responsible for high electrical conductivity. The discoverers of these polymers were awarded the Nobel Prize in Chemistry in 2000. Advantages of polymer conductors over conventional metallic conductors include low-cost production, easy processing using conventional technologies, better mechanical properties and the potential for manipulating their electrical and optical characteristics. Some have found applications in organic LEDs, solar cells, transistors or different types of sensors. However, the main disadvantage of existing conductive polymers is their low chemical and thermal stability, which is linked to the presence of foreign elements in their structure. A number of laboratories around the world are, therefore, trying to prepare new types of conductive polymers that do not contain such elements. It was the Czech-Spanish-Swiss team that was the first to succeed in this challenge.
“In our work, we studied what is known as πconjugated polymers, which are characterized by alternating simple and double bonds between carbon atoms. Nevertheless, on the basis of the polymer's internal stress compensation and its electron structure, an appropriate choice of the polymer's basic construction units can be made to prepare a one-dimensional system that is located near the phase transition. It was the use of the right starting molecules that produced the highly conductive polymer with free electrons without the necessary presence of foreign elements. This approach to the synthesis of 1D conductive polymers may lead to the development of a new generation of organic conductors for molecular electronics, says Jelínek.
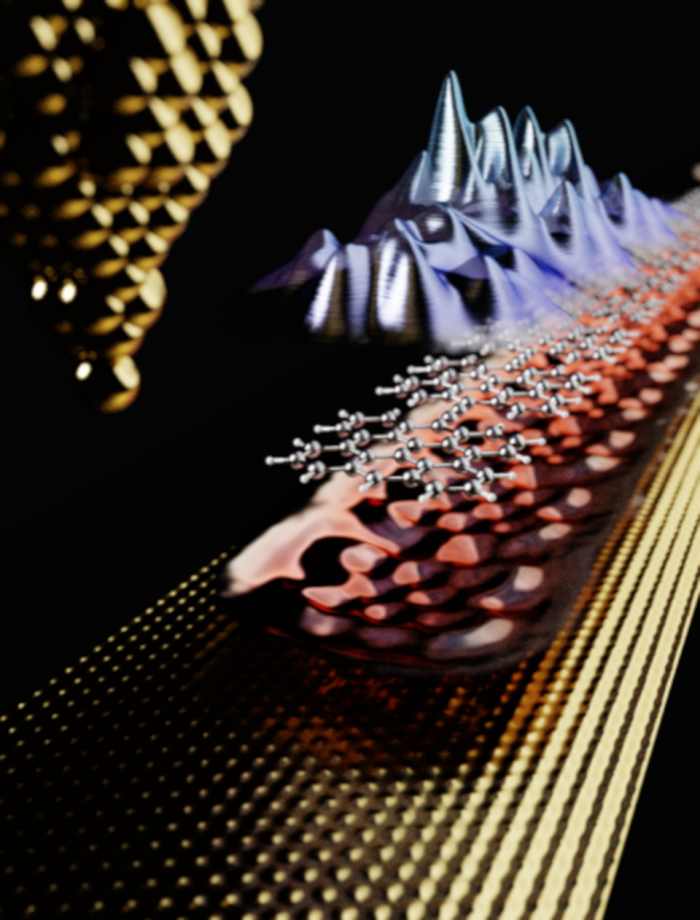
The synthesis of 1D polymer chains took place on a gold surface. The chemical structure and electrical properties were examined by scientists using a scanning microscope with a chemically modified tip that enabled imaging of individual molecules (Fig. 1) "Conductive polymers were prepared by applying appropriate molecules, developed by Spanish colleagues, to the gold surface. Their subsequent heat treatment led to the formation of long 1D chains without any structural disturbances. The basic building units of the polymers were interconnected carbon bridges. In addition, the electrical properties of 1D polymers can be tuned using just the right choice of basic construction units, moving towards the development of 1D organic semiconductors, for example. Such polymers could find applications not only in the development of molecular electronics, but also in new optoelectronic devices or organic solar cells," says Bruno de la Torre from both the FZU and RCPTM.
Following the collaboration of the Spanish and Czech teams, the results of the study have recently led to the development of chemical protocols for polymer synthesis, the preparation of which is not possible using normal processes.2 "The work in Nature Nanotechnology shows unique possibilities for surface chemistry, where different chemical rules are applied compared to reactions taking place in liquid or gaseous environments. This enables the preparation of completely unique materials such as 1D molecular conductors, with their conductivity resulting directly from their structure. These findings could help address a number of other scientific challenges and prepare a new generation of low dimensional structures with completely new optical, magnetic and electrical properties," adds Radek Zbořil from the RCPTM, Olomouc.
References:
1. B. Cirera, A. Sánchez-Grande, B. de la Torre, J. Santos, Sh. Edalatmanesh, E. Rodríguez-Sánchez, K. Lauwaet, B. Mallada, R. Zbořil, R. Miranda, O. Gröning, P. Jelínek, N. Martín, D. Ecija,Tailoring topological order and π-conjugation to engineer quasi-metallic polymers, Nature Nanotech. (2020) DOI: 10.1038/s41565-020-0668-7.
2. A. Sánchez-Grande, B. de la Torre, J. Santos, B. Cirera, K. Lauwaet, T. Chutora, S. Edalatmanesh, P. Mutombo, J. Rosen, R. Zbořil, R. Miranda, J. Björk, P. Jelínek, N. Martín and D. Écija," ANGEWANDTE CHEMIE INTERNATIONAL EDITION vol. 58, iss. 20, pp. 6559-6563, 2019. DOI: 10.1002/anie.201814154.
Contact person:
Pavel Jelínek
Fyzikální ústav AV ČR | RCPTM
E-mail: jelinekp [at] fzu [dot] cz | M: 734 353 740