Vědci z ÚOCHB k epidemii nového koronaviru v médiích
Publikace
Všechny publikace
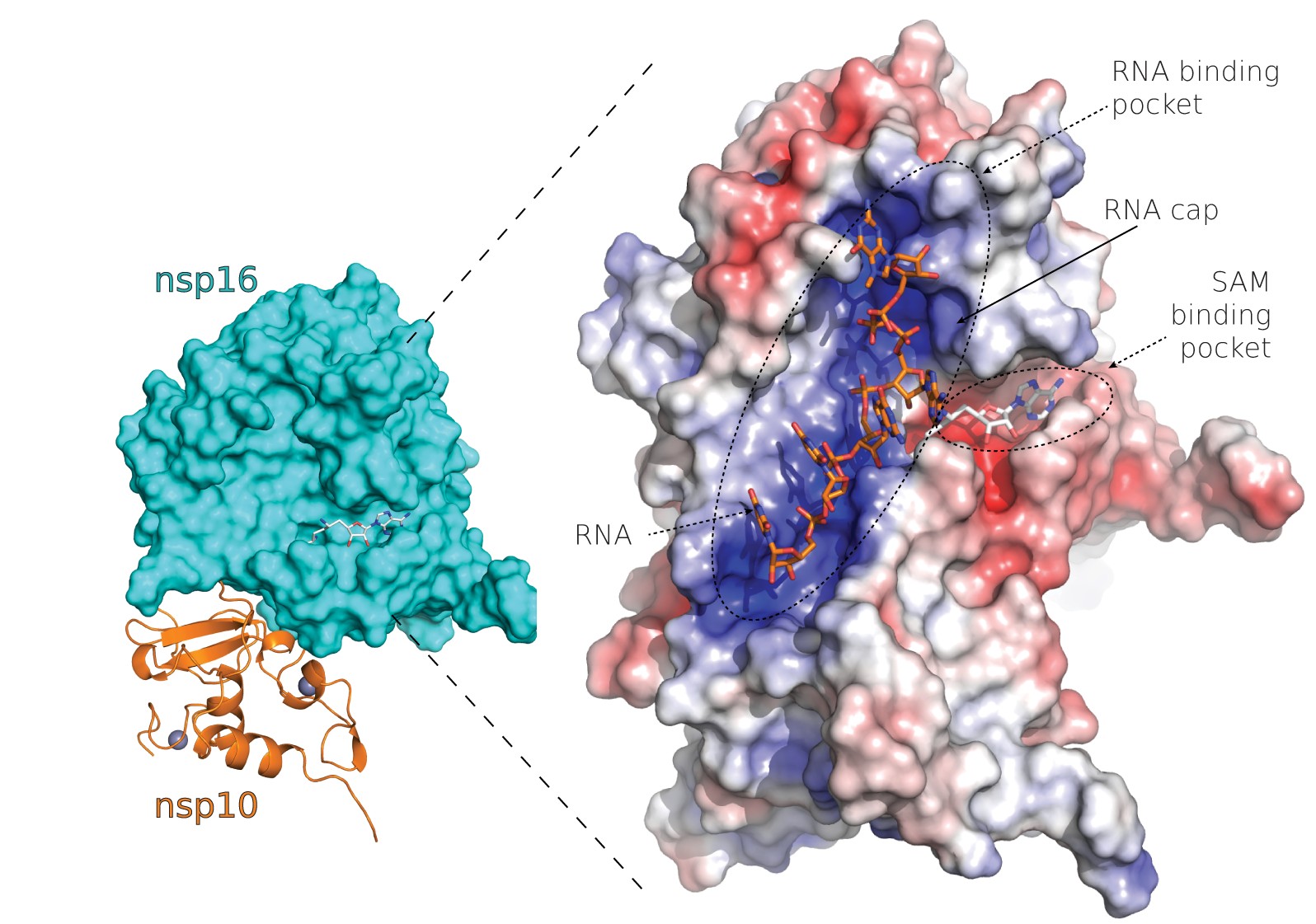
Structural analysis of the SARS-CoV-2 methyltransferase complex involved in RNA cap creation bound to sinefungin
Nature Communications 11 : 3717 (2020).
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of the COVID-19 pandemic. 2′-O-RNA methyltransferase (MTase) is one of the enzymes of this virus that is a potential target for antiviral therapy as it is crucial for RNA cap formation; an essential process for viral RNA stability. This MTase function is associated with the nsp16 protein, which requires a cofactor, nsp10, for its proper activity. Here we show the crystal structure of the nsp10-nsp16 complex bound to the pan-MTase inhibitor sinefungin in the active site. Our structural comparisons reveal low conservation of the MTase catalytic site between Zika and SARS-CoV-2 viruses, but high conservation of the MTase active site between SARS-CoV-2 and SARS-CoV viruses; these data suggest that the preparation of MTase inhibitors targeting several coronaviruses - but not flaviviruses - should be feasible. Together, our data add to important information for structure-based drug discovery.
Toll-like receptor dual-acting agonists are potent inducers of PBMC-produced cytokines that inhibit hepatitis B virus production in primary human hepatocytes
Scientific Reports 10 : 12767 (2020).
Mass spectrometry imaging of free-floating brain sections detects pathological lipid distribution in a mouse model of Alzheimer's-like pathology
Analyst 145 (13): 4595-4605 (2020).
Morphing radial molecular property functions of hydroxyl
Journal of Quantitative Spectroscopy and Radiative Transfer 254 : Early View (2020).
Relativistic Heavy-Neighbor-Atom Effects on NMR Shifts: Concepts and Trends Across the Periodic Table
Chemical Reviews 2020 : Early View (2020).