Signalling bias as the way to side-effect-free medication (22.7. 2020)
More than 30 % of currently marketed medications act via G-protein coupled receptors (GPCRs). This type of membrane receptors is involved in the control of a wide range of physiological processes, from the processing of sensory stimuli, through the regulation of behavior and mood, hormonal and immune responses, to the control of autonomic functions and cell proliferation. Thus, GPCRs represent one of the most important pharmacotherapeutic targets. In contrast to traditional agonists activating multiple signalling pathways, agonists biased towards a given signalling pathway represent a new generation of drugs with increased specificity and fewer adverse effects. Enormous research has been done on agonists biased towards either G-protein or arrestin mediated pathway.
In our laboratory we focus on the study of muscarinic acetylcholine receptors, which belong to the typical representatives of GPCR. Here, as a proof of concept, we demonstrate unprecedented signalling bias solely at the level of G‑protein-mediated signalling. We present agonists of muscarinic acetylcholine receptors, a member of GPCR family, that exclusively inhibit cAMP synthesis through activation of Gi‑protein pathway and thus are functionally selective for M2 and M4 receptor subtypes. Muscarinic receptors M2, M4 represent one of the pharmacological targets in the treatment of pain. Newly discovered agonists may lead to the development of new non-addictive analgesics such as opiates, or immunity-weakening as steroid analgesics. Such muscarinic analgesics would not cause side effects mediated by activation of the Gq protein signaling pathway, such as incontinence, excessive salivation and sweating, and more.

A) General scheme of coupling GPCRs with individual subtypes of G‑proteins or arrestins after activation by a traditional full agonist (left) and an agonist preferring Gi‑protein activation (right). AC, adenylyl cyclase; PLC, phospholipase C. B) Activation of M2 receptor by the traditional agonist (blue) resulting in stimulation of the Gi and Gs‑protein pathway leading to a decrease in cAMP level in the cell at low concentration of agonist and an increase in cAMP level at high concentrations of the agonist. Activation of M2 receptor by two different Gi‑biased agonists (red, orange) resulting in stimulation of the Gi‑protein pathway only and decrease in cAMP level.
Randáková A, Nelic D, Ungerová D, Nwokoye P, Su Q, Doležal V, El-Fakahany EE, Boulos J, Jakubík J. Novel M2-Selective, Gi-Biased Agonists of Muscarinic Acetylcholine Receptors. Br J Pharmacol. 2020 May;177(9):2073-2089. doi: 10.1111/bph.14970.
Lipokine 5-PAHSA is Regulated by Adipose Triglyceride Lipase and Primes Adipocytes for de novo Lipogenesis in Mice (22.7. 2020)
5-PAHSA belongs to newly discovered lipids which show a great promise as anti-diabetic and anti-inflammatory compounds, but their action is poorly explored. Animal and cell culture experiments showed that 5-PAHSA helps to import glucose into the cells in a different way from insulin. While insulin stimulates lipid accumulation, administration of 5-PAHSA results in the activation of energy-demanding metabolic processes of fatty acid synthesis. Therefore, glucose is used more efficiently.
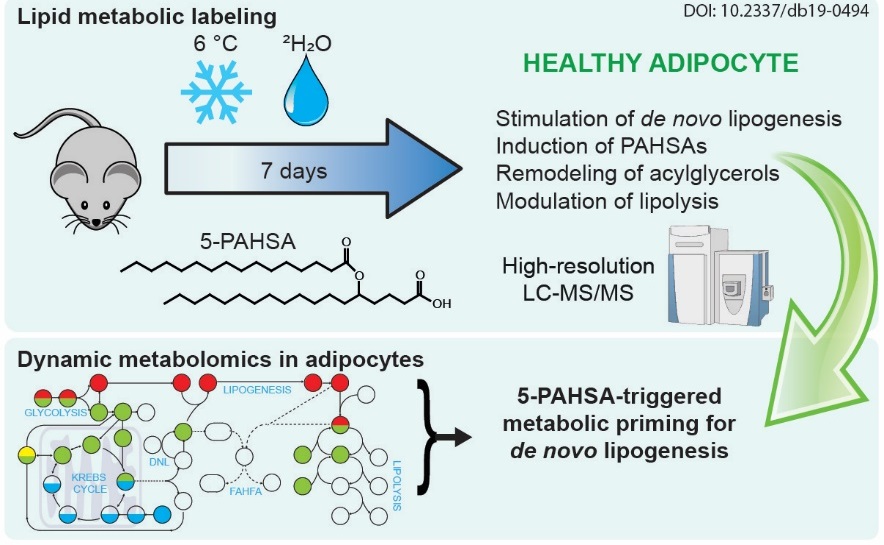
The effect of a bioactive lipid 5-PAHSA on glucose and lipid metabolism. Labeling of metabolic pathways in mice and advanced analytical methods were used to explore the beneficial effect of 5-PAHSA bioactive lipids in glucose utilization.
Lipokine 5-PAHSA is Regulated by Adipose Triglyceride Lipase and Primes Adipocytes for de novo Lipogenesis in Mice. Paluchova V, Oseeva M, Brezinova M, Cajka T, Bardova K, Adamcova K, Zacek P, Brejchova K, Balas L, Chodounska H, Kudova E, Schreiber R, Zechner R, Durand T, Rossmeisl M, Abumrad NA, Kopecky J, Kuda O. Diabetes. 2019 Dec 5. pii: db190494. doi: 10.2337/db19-0494.
Elucidation of TRPA1 activation mechanisms may help to understand the pathophysiology of chronic pain (3.2. 2020)
A plethora of serious diseases is followed by chronic pain the molecular mechanisms of which are not fully unraveled. The research on TRPA1 ion channel, which is involved in these mechanisms, is conducted by the researchers from the Laboratory of Cellular Neurophysiology of the Institute of Physiology CAS. In collaboration with the laboratory CBMN from the University of Bordeaux they proved that the activity of human TRPA1 is significantly regulated by membrane phospholipids at negative membrane potentials. They succeeded in identifying the binding site of TRPA1 channel, which interacts with phospholipids at low concentrations of calcium ions. At higher concentrations of calcium ions, the same region represents the binding site for a regulatory protein calmodulin, which modulates the TRPA1 channel in an activity-dependent manner. These new findings suggest that the correct phospholipid composition of the plasma membrane is important for TRPA1 activation under physiological conditions. Membrane phospholipids help to stabilize the interface between TRPA1 subunits to maintain the transduction of signals into the channel opening.
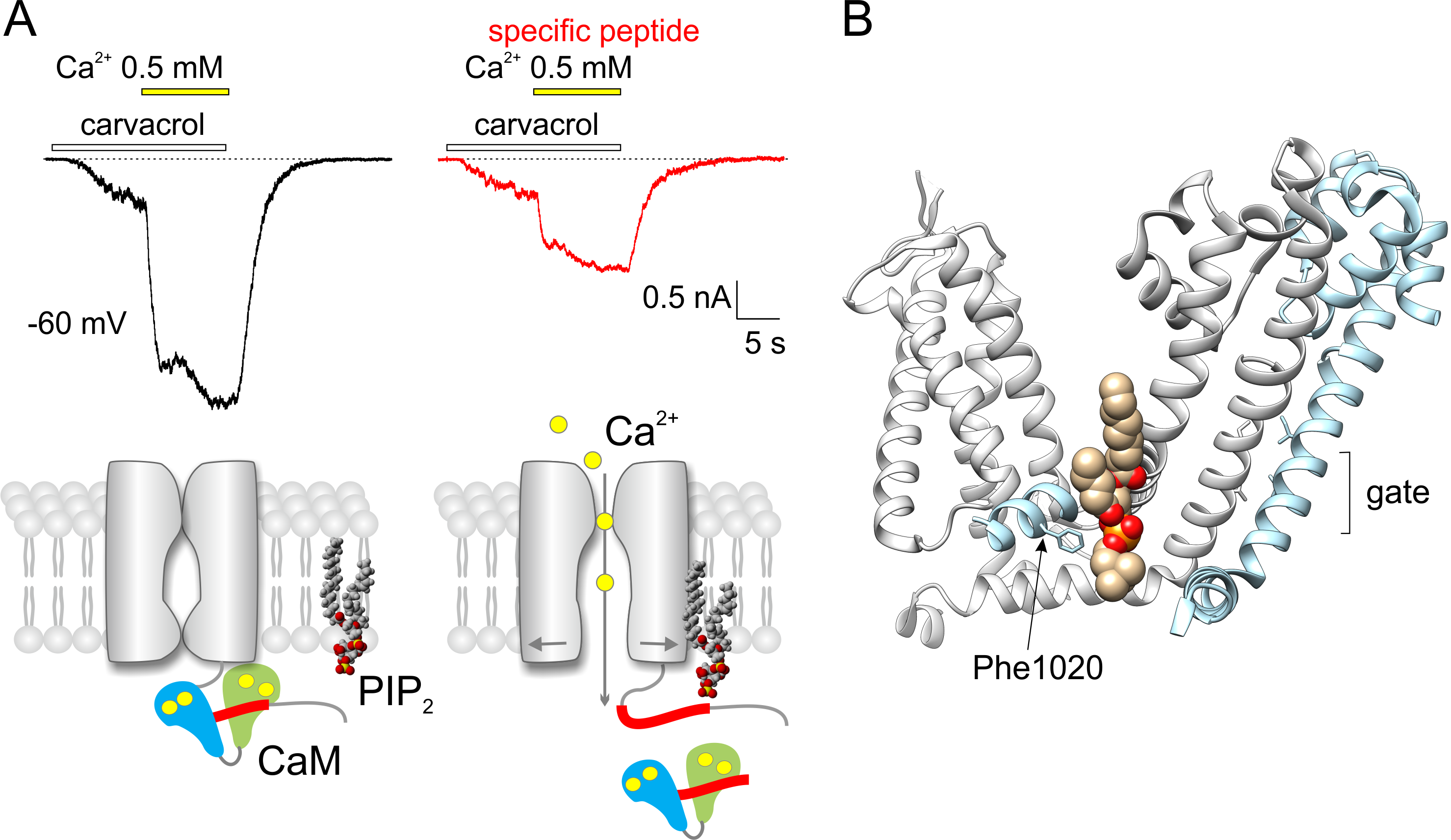
(A) Electrophysiological recordings of TRPA1 channel‘s responses to carvacrol (100 µM) and to elevated concentration of calcium ions (from 0 to 0.5 mM). Recordings from two F11 cells recorded with a pipette filled with control solution (left) and solution with the specific peptide T1003-P1034 added (right). Lower, there is a suggested mechanism of competition between calmodulin (CaM) and phospholipid (PIP2) for the binding site of TRPA1. (B) The binding site for phospholipids in the region of two neighboring subunits of TRPA1 channel. Only the transmembrane part of the protein, which contributes to the binding and transduction of signals into the channel gate, is shown (PDB code of the structure 6pqq). The role of phenylalanine Phe1020 in the interaction was revealed in the study.
MACIKOVA L, SINICA V, KADKOVA A, VILLETTE S, CIACCAFAVA A, FAHERTY J, LECOMTE S, ALVES ID, VLACHOVA V: Putative interaction site for membrane phospholipids controls activation of TRPA1 channel at physiological membrane potentials. The FEBS Journal 286: 3664-3683, 2019.
Innovative method of the Forhkead transcription factor FOXO3 regulation (29.1. 2020)
The study published in prestigious journal eLife identifies small molecule compounds that interact with the Forkhead box O3 transcription factor (FOXO3) and modulate its activity.
FOXO3, with the characteristic fork head DNA-binding domain is part of the O subclass of the forkhead family of transcription factors. These transcription factors have important roles in mammalian cells in regards to regulating cell homeostasis, differentiation, longevity and steer cell death. The activity of FOXO3 in particular contributes to therapy-resistance programs that protect cancer cells during chemo and radiotherapy. Recent studies have also found the DNA-binding domain (DBD) of FOXO aid protein-protein interactions with other key regulators of longevity and death, and drug resistance. A reversible inhibition of FOXO3 activity by small compounds thereby might boost anti-tumor immune responses.
In order to inhibit the FOXO3 activity, it was first necessary to identify small molecule compounds that could block the interaction between FOXO3 and DNA. Using the structural data of FOXO3 DBD and FOXO4 DBD, the researchers developed six different pharmacophore models that were used for in silico screening of small molecule compound databases. Selected compounds were then tested for their ability to inhibit FOXO3 function both in vitro, and in cancer cell lines. The interactions of these compounds with FOXO3 DBD were assessed using NMR spectroscopy and docking studies.
The teams of Dr. V. Obšilová from the Institute of Physiology CAS in BIOCEV and prof. T. Obšil from the Faculty of Science, Charles University with the team of prof. M. J. Ausserlechner from the Medical University of Innsbruck, Austria identified the compounds S9 and its oxalate salt S9OX as compounds able to inhibit the FOXO3 activity in cancer cells. They also proposed that due to their mode of binding to FOXO3 DBD, these compounds may also interfere with protein-protein interactions of FOXO3. The advantage of these compounds is the strict control of application-dose and -time and the fact that they are not immunogenic allowing repeated applications – so dose- and application time can be adjusted to damage cancer cells or boost anti-cancer immunity, but also limit unwanted side effects of FOXO-inhibition on stem cells and other somatic tissues. Future research will be focused on investigation whether or not S9 can be used as a chemical foundation for developing FOXO-regulatory compounds that would control the functions and target gene subsets of FOXO transcription factors.

Compounds S9 blocks the DNA binding surface of Forkhead transcription factor FOXO3. The figure shows the structural model of the DNA-binding domain of FOXO3 with bound compound S9 based on data from NMR measurements and docking simulations.
Hagenbuchner, J.+, Obšilová, V.+, Kaserer, T.+, Kaiser, N., Rass, B., Pšenáková, K., Dočekal, V., Alblová, M., Kohoutová, K., Schuster, D., Aneichyk, T., Veselý, J., Obexer, P., Obšil, T.*, Ausserlechner, M. J.* Modulating FOXO3 transcriptional activity by small, DBD-binding molecules. eLife. 2019, 8(Dec 4), e48876. doi: 10.7554/eLife.48876
Transcription factor Hif-1a is required for the correct development of the sympathetic nervous system and innervation of the heart (16.1. 2020)
Hypoxia-inducible factor 1 (Hif-1) is the master regulator of transcriptional responses of cells to decreased oxygen availability. Research teams of the Institutes of Biotechnology and Physiology CAS and the 1st Medical Faculty UK showed that genetic mutation of the Hif-1a gene suppresses the embryonic development of preganglionic and postganglionic neurons of the sympathetic nerve system and negatively affects sympathetic innervation of the heart that plays a primary role in the regulation of heart rate and contractility. Mice with conditional deletion of Hif-1a gene exhibited coronary artery anomalies and decreased cardiac contractile function. These data indicate that deregulation of the transcription factor Hif-1a can result in serious cardiovascular diseases associated with the autonomic nervous system dysbalance and open the way to a development of new therapeutic strategies.
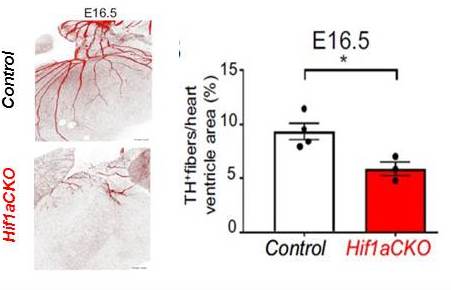
Impaired sympathetic innervation of hearts with Hif-1a deletion (Hif1aCKO): Immunohistochemical staining of tyrosine hydroxylase (TH) in posterior view of hearts, and quantification of TH-positive fibers per ventricle area in E16.5 control and Hif1aCKO littermates.
Bohuslavová, Romana - Čerychová, Radka - Papoušek, František - Olejníčková, Veronika - Bartoš, M. - Goerlach, A. - Kolář, František - Sedmera, David - Semenza, G.L. - Pavlínková, Gabriela: HIF-1 alpha is required for development of the sympathetic nervous system. Proceedings of the National Academy of Sciences of the United States of America. Roč. 116, č. 27 (2019), s. 13414-13423. ISSN 0027-8424, IF: 9.580, 2018. DOI: 10.1073/pnas.1903510116
Load next




