Lithium-ion batteries and metal hydrides are key technologies for storing green energy.
Lithium-ion battery: Rechargeable lithium-ion batteries are used in mobile devices like cell phones and laptops. For applications in electric or hybrid vehicles the efficiency, capacity and safety must be improved considerably. To this end a detailed understanding of the involved chemical and physical phenomena is mandatory.
During discharging of a lithium-ion battery, lithium atoms reversibly will be stored on interstitial lattice sites of the crystals of a many-particle electrode.
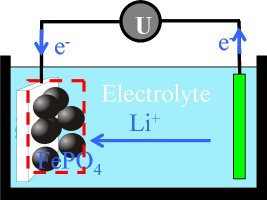
Figure 1: Sketch of a lithium-ion battery (LiFePO4).
During charging and discharging of a LiFePO4 battery the lithium atoms form a two phase system with a lithium-rich and a lithium-poor phase. The objective is to find a suitable description of the underlying effects and to simulate the hysteresis in the capacity-voltage diagram.
Scenario A: All FePO4 particles behave identical. This implies that the phase transition simultaneously occurs in all particles implying that the battery can be described by a single particle model, i.e. the Core-Shell-Model.

Figure 2: Simulation of lithium storage within a single cathode particle (LiFePO4), Core-Shell-Model.
Simulations show that a single particle model cannot correctly predict the correct capacity-voltage diagram for slow charging/discharging processes.
Scenario B: The particles behave differently. The phase transition occurs within in the particle ensemble. On that principle a stochastic many-particle model and an equivalent Fokker-Planck-Model is developed. Both models correctly predict the voltage-capacity-diagrams for large and low charging/discharging times as well. Moreover the models allow the simulation of the phase transition within the ensemble for varying particle size distribution.

Figure 3: Simulation of the voltage-capacity-diagram. blue: Fokker-Planck-model, red: SDE-Model, grey: chemical potential single particle.
Metal hydride: The application of fuel cells in automobiles relies on safe and effective hydrogen storage. Hydrogen can be stored as liquid or vapor in pressure vessels, on large surfaces of nanostructures or in metallic crystals. Concerning the volume density of hydrogen, hydrogen storage in metallic crystals is the most effective one.

Figure 4: Visualization of hydrogen storage by magnesium particles.
During the reversible storage of hydrogen in magnesia crystals a hydride phase will be formed. This process and its inversion is accompanied by phase transition and hysteresis. An active research goal is the modeling and simulation of the hydration and dehydration processes.
 |  | |
| Figure 4: Simulation of a loading/unloading process with a many particle model. Left: 10 storage particles. Right: 1000 storage particles. | ||
Publications
 Articles in Refereed Journals
Articles in Refereed Journals
-
W. Dreyer, P. Friz, P. Gajewski, C. Guhlke, M. Maurelli, Stochastic many-particle model for LFP electrodes, Continuum Mechanics and Thermodynamics, 30 (2018), pp. 593--628, DOI 10.1007/s00161-018-0629-7 .
Abstract
In the framework of non-equilibrium thermodynamics we derive a new model for porous electrodes. The model is applied to LiFePO4 (LFP) electrodes consisting of many LFP particles of nanometer size. The phase transition from a lithium-poor to a lithium-rich phase within LFP electrodes is controlled by surface fluctuations leading to a system of stochastic differential equations. The model is capable to derive an explicit relation between battery voltage and current that is controlled by thermodynamic state variables. This voltage-current relation reveals that in thin LFP electrodes lithium intercalation from the particle surfaces into the LFP particles is the principal rate limiting process. There are only two constant kinetic parameters in the model describing the intercalation rate and the fluctuation strength, respectively. The model correctly predicts several features of LFP electrodes, viz. the phase transition, the observed voltage plateaus, hysteresis and the rate limiting capacity. Moreover we study the impact of both the particle size distribution and the active surface area on the voltagecharge characteristics of the electrode. Finally we carefully discuss the phase transition for varying charging/discharging rates. -
W. Dreyer, C. Guhlke, R. Müller, A new perspective on the electron transfer: Recovering the Butler--Volmer equation in non-equilibrium thermodynamics, Physical Chemistry Chemical Physics, 18 (2016), pp. 24966--24983, DOI 10.1039/C6CP04142F .
Abstract
Understanding and correct mathematical description of electron transfer reaction is a central question in electrochemistry. Typically the electron transfer reactions are described by the Butler-Volmer equation which has its origin in kinetic theories. The Butler-Volmer equation relates interfacial reaction rates to bulk quantities like the electrostatic potential and electrolyte concentrations. Since in the classical form, the validity of the Butler-Volmer equation is limited to some simple electrochemical systems, many attempts have been made to generalize the Butler-Volmer equation. Based on non-equilibrium thermodynamics we have recently derived a reduced model for the electrode-electrolyte interface. This reduced model includes surface reactions but does not resolve the charge layer at the interface. Instead it is locally electroneutral and consistently incorporates all features of the double layer into a set of interface conditions. In the context of this reduced model we are able to derive a general Butler-Volmer equation. We discuss the application of the new Butler-Volmer equations to different scenarios like electron transfer reactions at metal electrodes, the intercalation process in lithium-iron-phosphate electrodes and adsorption processes. We illustrate the theory by an example of electroplating. -
W. Dreyer, R. Huth, A. Mielke, J. Rehberg, M. Winkler, Global existence for a nonlocal and nonlinear Fokker--Planck equation, ZAMP Zeitschrift fur Angewandte Mathematik und Physik. ZAMP. Journal of Applied Mathematics and Physics. Journal de Mathematiques et de Physique Appliquees, 66 (2015), pp. 293--315.
Abstract
We consider a Fokker-Planck equation on a compact interval where, as a constraint, the first moment is a prescribed function of time. Eliminating the associated Lagrange multiplier one obtains nonlinear and nonlocal terms. After establishing suitable local existence results, we use the relative entropy as an energy functional. However, the time-dependent constraint leads to a source term such that a delicate analysis is needed to show that the dissipation terms are strong enough to control the work done by the constraint. We obtain global existence of solutions as long as the prescribed first moment stays in the interior of an interval. If the prescribed moment converges to a constant value inside the interior of the interval, then the solution stabilises to the unique steady state. -
W. Dreyer, C. Guhlke, R. Müller, Overcoming the shortcomings of the Nernst--Planck model, Physical Chemistry Chemical Physics, 15 (2013), pp. 7075--7086, DOI 10.1039/C3CP44390F .
Abstract
This is a study on electrolytes that takes a thermodynamically consistent coupling between mechanics and diffusion into account. It removes some inherent deficiencies of the popular Nernst-Planck model. A boundary problem for equilibrium processes is used to illustrate the new features of our model. -
W. Dreyer, C. Guhlke, R. Huth, The behavior of a many-particle cathode in a lithium-ion battery, Physica D. Nonlinear Phenomena, 240 (2011), pp. 1008--1019.
-
W. Dreyer, M. Gaberšček, C. Guhlke, R. Huth, J. Jamnik, Phase transition and hysteresis in a rechargeable lithium battery, European Journal of Applied Mathematics, 22 (2011), pp. 267--290.
-
W. Dreyer, C. Guhlke, M. Herrmann, Hysteresis and phase transition in many-particle storage systems, Continuum Mechanics and Thermodynamics, 23 (2011), pp. 211--231.
Abstract
We study the behavior of systems consisting of ensembles of interconnected storage particles. Our examples concern the storage of lithium in many-particle electrodes of rechargeable lithium-ion batteries and the storage of air in a system of interconnected rubber balloons. We are particularly interested in those storage systems whose constituents exhibit non-monotone material behavior leading to transitions between two coexisting phases and to hysteresis. In the current study we consider the case that the time to approach equilibrium of a single storage particle is much smaller than the time for full charging of the ensemble. In this regime the evolution of the probability to find a particle of the ensemble in a certain state, may be described by a nonlocal conservation law of Fokker-Planck type. Two constant parameter control whether the ensemble transits the 2-phase region along a Maxwell line or along a hysteresis path or if the ensemble shows the same non-monotone behavior as its constituents. -
W. Dreyer, J. Jamnik, C. Guhlke, R. Huth, J. Moškon, M. Gaberšček, The thermodynamic origin of hysteresis in insertion batteries, Nature Materials, 9 (2010), pp. 448--453.
 Talks, Poster
Talks, Poster
-
M. Maurelli , A McKean--Vlasov SDE with reflecting boundaries, CASA Colloquium, Eindhoven University of Technology, Department of Mathematics and Computer Science, Netherlands, January 10, 2018.
-
W. Dreyer, J. Fuhrmann, P. Gajewski, C. Guhlke, M. Landstorfer, M. Maurelli, R. Müller, Stochastic model for LiFePO4-electrodes, ModVal14 -- 14th Symposium on Fuel Cell and Battery Modeling and Experimental Validation, Karlsruhe, March 2 - 3, 2017.
-
P. Gajewski, M. Maurelli, Stochastic methods for the analysis of lithium-ion batteries, Matheon Center Days, April 20 - 21, 2015, Technische Universität Berlin, April 21, 2015.
-
C. Guhlke, Hysteresis due to non-monotone material behaviour inside many-particle systems, SIAM Conference on Mathematical Aspects of Materials Science (MS10), May 23 - 26, 2010, Philadelphia, USA, May 23, 2010.
-
C. Guhlke, Hysteresis due to non-monotone material behaviour inside many-particle systems, DPG Spring Meeting 2010, March 21 - 26, 2010, Regensburg, March 25, 2010.
-
W. Dreyer, Hysteresis and phase transition in many-particle storage systems, 13th International Conference on Hyperbolic Problems: Theory, Numerics, Applications (HYP 2010), June 14 - 19, 2010, Beijing, China, June 17, 2010.
-
W. Dreyer, On a paradox within the phase field modeling of storage systems and its resolution, 8th AIMS International Conference on Dynamical Systems, Differential Equations and Applications, May 25 - 28, 2010, Technische Universität Dresden, May 26, 2010.
-
W. Dreyer, On a paradox within the phase field modeling of storage systems and its resolution, PF09 -- 2nd Symposium on Phase-Field Modelling in Materials Science, August 30 - September 2, 2009, Universität Aachen, Kerkrade, Netherlands, August 31, 2009.
-
W. Dreyer, Phase transitions and kinetic relations, Séminaire Fluides Compressibles, Université Pierre et Marie Curie, Laboratoire Jacques-Louis Lions, Paris, France, September 30, 2009.
-
W. Dreyer, Phase transitions during hydrogen storage and in lithium-ion batteries, EUROTHERM Seminar no. 84: Thermodynamics of Phase Changes, May 25 - 27, 2009, Université Catholique de Louvain, Namur, Belgium, May 27, 2009.