The paper provides new insights into the modulatory role of endocannabinoid signaling during the light entrainment of the SCN.
Suprachiasmatic nucleus (SCN) of the hypothalamus is the master clock that drives circadian rhythms in physiology and behavior and adjusts their timing to external cues. Neurotransmitter glutamate and glutamatergic receptors sensitive to N-methyl-D-aspartate (NMDA) play a dual role in the SCN by coupling astrocytic and neuronal single cell oscillators and by resetting their phase in response to light. Recent reports suggested that signaling by endogenous cannabinoids (ECs) participates in both of these functions. ECs, such as 2-arachidonoylglycerol (2-AG), act via presynaptic CB1 receptors to modulate synaptic activity and affect the SCN response to light-mimicking NMDA stimulus in a time-dependent manner. We demonstrate that circadian clock in the rat SCN regulates expression of 2-AG transport, synthesis and degradation enzymes as well as its receptors. Inhibition of the major 2-AG synthesis enzyme, diacylglycerol lipase, enhances the phase delay and lowers the amplitude of explanted SCN rhythm in response to NMDAR activation. Using PER2 bioluminescence imaging, we show how single cell oscillators reflect the phase response of the whole SCN. Additionally, we present strong evidence that the zero amplitude behavior of the SCN in response to single NMDA stimulus in the middle of subjective night is the result of a loss of rhythm in individual SCN cells. The paper provides new insights into the modulatory role of endocannabinoid signaling during the light entrainment of the SCN.

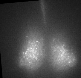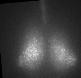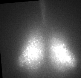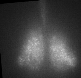
Fig 1. Explanted SCN from mPer2Luc mouse oscillating in vitro was treated by diacylglycerol lipase inhibitor and NMDA during daytime (left) or in the middle of subjective nighttime (right).
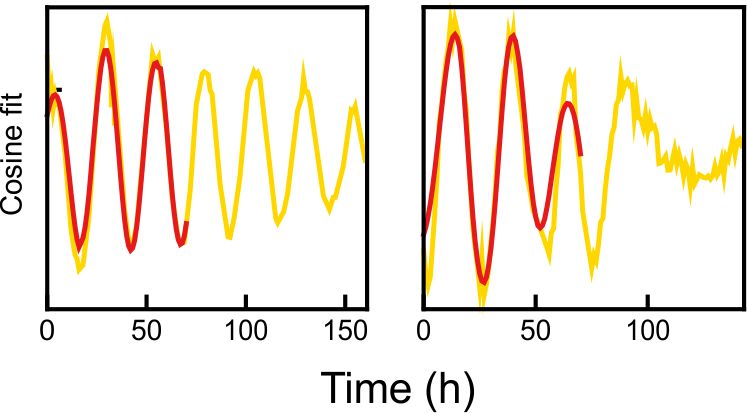
Fig 2. PER2 levels in a single representative neuron from the explants shown in Fig. 2. Red line shows the signal before treatment.