The intracellular concentration of potassium and sodium cations, as well as of protons, is strictly regulated via the activity of a series of membrane proteins that mediate the flux of cations and protons with various transport mechanisms. Incorrect functioning of some transporters results in serious disorders and diseases. We study in detail the roles of individual transporters and the impact of their activity on cell fitness.
Potassium cations are crucial for many physiological processes (e.g. for negative charges compensations in macromolecules, for the regulation of intracellular pH, membrane potential or cell volume). On the other hand, increased levels of other alkali-metal cations (Li+, Na+, Rb+) are toxic for cells. For the maintenance of optimal intracellular cation concentrations in the cytosol, cells use a whole range of transporters whose perfect coordination ensure the cell a stable intracellular environment indispensable for the maintenance of life.
Yeast S. cerevisiae possesses more than ten different transporters for cations K+, Na+ and H+ localized in the plasma membrane (ensuring import and export of cations from cells) or in membranes of intracellular organelles (participating in the transport of cations among the cytosol and cell organelles). By characterization of particular transporters, we try to understand how eukaryotic cells adapt to continuous changes of ions concentrations in their extracellular environment.
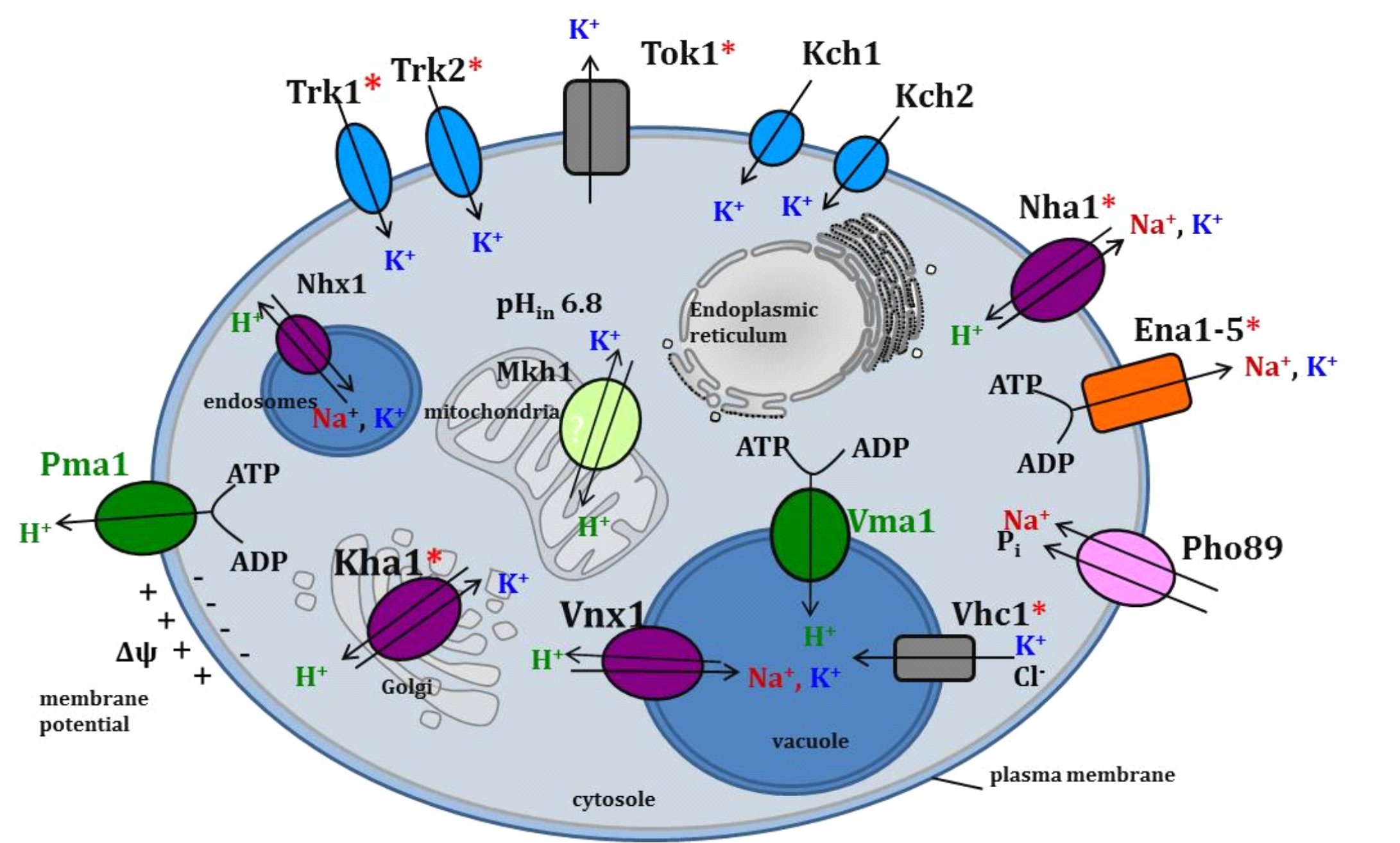
Transport systems for K+ and Na+ in the yeast S. cerevisiae (* transport systems in which characterization we have participated).
Studied proteins are tagged with fluorescent markers and cells producing these proteins are visualized by fluorescent microscope.
 |
 |
 |
||||
| Nha1-GFP in plasma membrane (Kinclová et al., 2001) |
Vhc1-GFP in vacuolar membrane (Petrezselyová et al., 2013) |
Kha1-GFP in the Golgi apparatus (Marešová et al., 2006) |
Examples of transporters’ localization in S.cerevisiae
By using mutagenesis, we search for amino-acid residues important for the transporter function.

Structural (3D) model of the transmembrane part of Na+/H+ antiporter Sod2-22 from Z. rouxiibased on E. coli NhaA antiporter structure (Kinclova-Zimmermannova et al., 2015). (a) General side view of the model. Highlighted residues Thr141, Ala179, Phe180 and Val375 are important for the determination of substrate specificity of the transporter. (b) Detailed view of hydrophobic cation filter important for the determinaton of substrate specificity of yeast Na+/H+ antiporters. The 3D model is observed from the top among helices 2, 4, 5 and 11. The putative cation binding residue (Asp176) placed behind the hydrophobic filter is also shown.
Characterization of the family of yeast plasma membrane Nha/Sod antiporters.
The family of yeast plasma membrane Na+/H+ antiporters includes transporters that transport all alkali-metal cations (Li+, Na+, K+, Rb+), but also systems that recognize only Li+ and Na+ (Kinclová et al., 2002). We have identified amino-acid residues in the 4th (Thr141, Pro145 and Ser150), 5th (Ala179) and 11th (Val375) transmembrane domain (TMS) important for recognition, binding and transport of cations. Particular mutations of these residues resulted in a changed/broader substrate specificity for K+ cations in the Sod2-22 antiporter from Zygosaccharomyces rouxii (Kinclova-Zimmermannova, 2005; 2006; 2015). We created 3D-model of Sod2-22 transmembrane part that revealed that amino-acid residues identified by mutagenesis form a hydrophobic filter in the putative cation pathway’s core which is important for the determination of substrate specificity of Na+/H+ antiporters (Kinclová-Zimmermannová et al., 2015). Currently, we try to identify other protein regions that determine substrate specificity and transport activity in yeast Nha/Sod antiporters.
We determine transporter substrate specificity and transport activity.

Phylogenetic tree shows relation among yeast plasma membrane Na+/H+ antiporters that have been studied in our laboratory. Blue highlights antiporters recognizing as their substrates only or with a significantly higher affinity Na+ and Li+ cations; they primary detoxify yeast cells. Red highlights antiporters exporting potassium cations whose function in cell physiology is more complex – they participate in the regulation of intracellular K+, internal pH and cell volume.
At the level of gene expression, the biogenesis of particular protein or the interaction with other proteins.
Genes for particular transporters can be deleted in S. cerevisiae cells. Our laboratory possesses a large collection of yeast strains with deletions of genes for single transport system or with combinations of these deletions. Currently, we search for new regulatory mechanisms that participates in the regulation of cation and pH homeostasis in eukaryotic cells, e.g. at the level of transporters’ biogenesis (Rosas-Santiago et al., 2015; Rosas-Santiago et al., 2016), via tight connection among transporters of protons, potassium and/or calcium cations (Zimmermannova et al., 2015; Papouskova et al., 2015) or via the composition of cell membranes (Kodedová et al., 2015). Yeast strains lacking their own transport systems can also be used to study transporters from other organisms (plant, animal or human).
Study of plant and animal transporters expressed in yeasts
Maintenance of stable intracellular ion concentration is also fundamental for plant and animal cells. In plant cells, mainly Na+/H+ antiporters localized in the plasma membrane and in the vacuolar membrane participate in the maintenance of low Na+ concentration in the cytosol. Yeast mutant strains lacking their own transporters for alkali-metal cations were used for the characterization of transport properties of plant OsNhx1 transporter from rice (Oryza sativa; Kinclová et al., 2004). We have found that OsNhx1 is an antiporter with broad substrate specificity for cations Li+, Na+, K+ and Rb+. Characterization of another transporter, AtChx17 from Arabidopsis thaliana, in yeasts revealed that it is an K+/H+ antiporter localized in the Golgi apparatus that participates in the regulation of internal content of K+ and protons (Marešová et al., 2006). Both plant antiporters were able to functionally replace their yeast homologs.
Expression of OsNhx1 antiporter from rice in yeasts improved the cell growth in the presence of high concentrations of cations Na+, Li+, K+, Rb+, but not Cs+, with respect to its substrate specificity. The antiporter was expressed in the S. cerevisiae strain AW22 that lacks genes encoding antiporters Nha1 and Nhx1 and sodium ATPases Ena under the control of a weak promoter (pNHA1OsNHX1) or a strong promoter (pPMA1OsNHX1).
Study of human NHA2 protein: The human antiporter NHA2 is specifically expressed in osteoclasts where it is involved in cell differentiation. Its insufficiency causes serious diseases. Upon its heterologous expression in a S. cerevisiae strain lacking its own Na+ exporters, we have identified, in collaboration with scientists from the USA and China, several amino-acid residues important for its transport activity (Huang et al., 2010).
Characterization of mammalian potassium channels. S. cerevisiae were also used to express and characterize mammalian K+-channels, such as Kir2.1 (Koláčná et al., 2005) whose mutation is connected to Andersen syndrome affecting the heart in human, or EAG1 (Schwarzer et al., 2008), which is highly expressed in the brain and whose overexpression may favour tumor cell proliferation.