Targeting enzyme exosites by in-situ click chemistry: New strategy for anticancer drug design (ExoClick)
PI: Pavlína Maloy Řezáčová, co-PIs: Milan Vrábel, Kvido Stříšovský, Michael Mareš, Pavel Majer
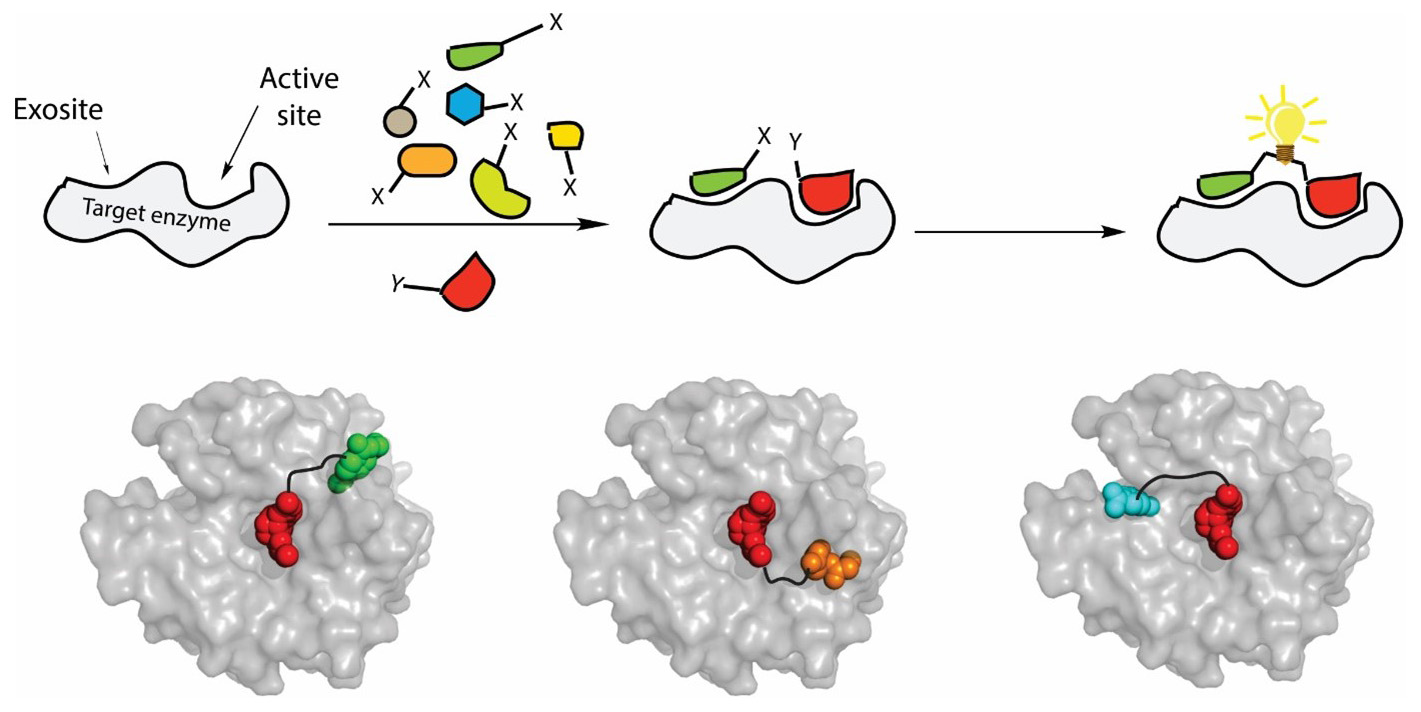
In the proposed program we develop a new approach for the regulation of cancer-associated enzymes by bivalent exosite-targeting inhibitors. To this end, we employ in situ click chemistry, a powerful method for drug lead discovery based on protein target-guided assembly of small building blocks (SBB). Broad SBB diversity libraries (peptides, glycans, lipids, and their mimetics) will be used to select ligands for enzyme exosites by in situ click-conjugation with known active-site ligands. This will generate hybrid bivalent ligands displaying high affinity and selectivity driven by the synergy of both interaction regions. Using this strategy, we will design novel effective inhibitors of three groups of cancer-associated enzymes: carbonic anhydrases, ECM-degrading pericellular proteases, and intramembrane proteases. The structural binding mode of the inhibitors will be determined by crystallography and/or NMR and their binding properties will be improved by rational design. This interdisciplinary program integrates research of five IOCB groups covering organic chemistry, biochemistry and structural biology.
The program will yield (i) generally applicable technology for the discovery and targeting of enzyme exosites, (ii) important information about structure-inhibition relationships and interaction hotspots in several cancer-relevant enzymes, (iii) new research tools for target validation, and (iv) novel classes of lead compounds for cancer treatment.




