Pokrok ve studiu kadmia
 Cadmium is a toxic metal widely distributed in the environment, often due to human activity. Natural cadmium is composed of eight isotopes, and their proportions may vary slightly in different environmental compartments. This is due to a phenomenon called isotopic fractionation. Modern analytical methods allow these subtle variations to be measured, which can be useful in investigating the behaviour of cadmium in the environment. The researchers of the Institute of Geology of the Czech Academy of Sciences have established a method for measuring Cd isotopes in biological samples using thermal ionization mass spectroscopy (TIMS).
Cadmium is a toxic metal widely distributed in the environment, often due to human activity. Natural cadmium is composed of eight isotopes, and their proportions may vary slightly in different environmental compartments. This is due to a phenomenon called isotopic fractionation. Modern analytical methods allow these subtle variations to be measured, which can be useful in investigating the behaviour of cadmium in the environment. The researchers of the Institute of Geology of the Czech Academy of Sciences have established a method for measuring Cd isotopes in biological samples using thermal ionization mass spectroscopy (TIMS).
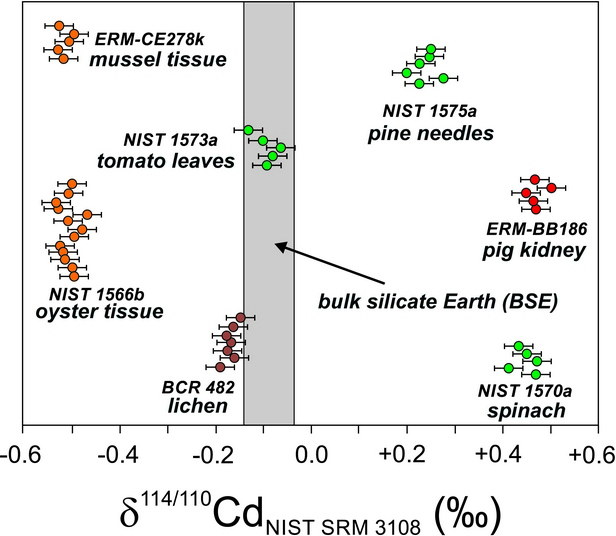
Isotopic composition of Cd in a sample is usually related to the isotopes 110Cd and 114Cd. It is expressed as a deviation (δ110/114Cd) from the value of an internationally recognized standard, which has δ110/114Cd value of zero by definition. Negative values of δ110/114Cd therefore indicate an enrichment with a lighter Cd isotope compared to this standard and vice versa.
By analysing a diverse set of samples of biogenic certified natural materials, we demonstrated a relatively wide range of isotopic variation of Cd. As can be seen from the figure, the δ110/114Cd values in the studied materials were significantly different. Strikingly negative values were observed in marine organisms (oysters and mussels), which were enriched with the lighter isotope. In contrast, a heavier Cd isotope enrichment was found in spinach leaves and pig kidney samples.
The obtained results published in the renowned analytical journal Talanta represent a useful tool in quality control of chemical analyses for all laboratories dealing with Cd isotopic analysis.