Scientists from the Laboratory of Biophysics at the Department of Optical and Biophysical Systems contributed to the field of DNA nanotechnology with a recent systematic analysis. The authors, led by Oleg Lunov, have summarized the current state of knowledge about the interactions of DNA nanostructures (DN) with cells and identified the key challenges from the point of view of cell biology that need to be addressed on the way to their clinical use. The analysis was published by Acta Biomaterialia. The work was written as part of ongoing collaboration with the team of prof. Nicholas Stephanopoulos from the Arizona State University.
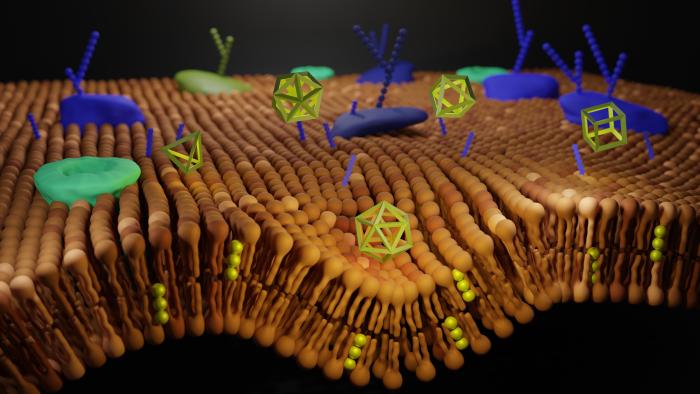
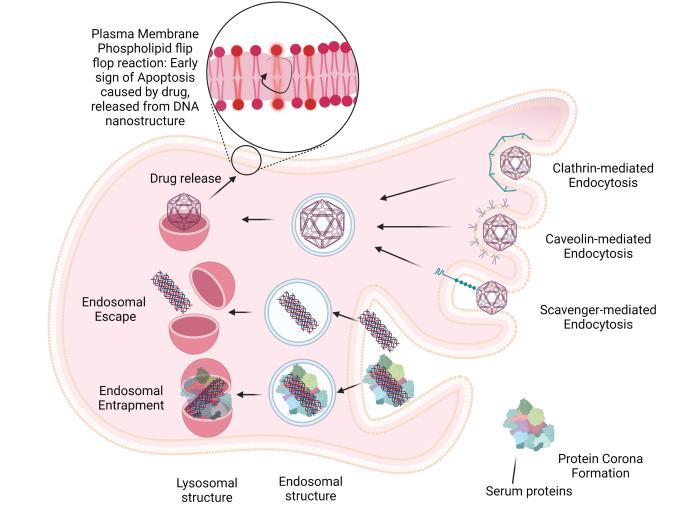
The authors had already discovered how the protein coating affects functioning of DN inside cells. In their latest article, in addition to studying the protein coating, they also focused on analysing the interaction of DN ligands with cell surface proteins. More specifically, on how they bind to surrounding cells, on cytotoxicity and DN interaction with living cells.
"DNA nanostructures have great biomedical potential. We expect to see a number of applications with them transferred into clinical use in the near future. However, what is essential for the clinical success of DNA nanostructures is understanding the challenges and limitations of their therapeutic application. This critical analysis of ours will help researchers create a plan to overcome them," said Oleg Lunov, head of the research team.
The latest overview prepared by the team from the Laboratory of Biophysics identifies some challenges in the field of DN, including their stability under physiological conditions or the importance of a cell model selection, i.e. the type and line of cells used in the research. Furthermore, it points out the necessity of unifying the rules in the preparation of DN, their characterization and experiments with them, as well as the need to use in vivo models that bring more relevant results in case of their future use in human medicine. It also talks about sequestration of the liver (separation of the dead part of the tissue from the living one). The liver is specifically mentioned because the vast majority of nanomaterials are eliminated in it.
“I consider the engagement of one of our teams in collaboration with a prestigious American institution in cutting-edge interdisciplinary research to be an outstanding achievement. It turns out that the interdisciplinarity that we bet on at the Division of Optics is paying off and our research is reaching a world-class level. The interconnection of physics, chemistry and biology has the potential to change the way we will look at science in the future and to improve our daily lives in a number of ways," says Alexandr Dejneka, head of the Division of Optics, to which the Laboratory of Biophysics belongs.
Nanostructures engineered from DNA can act both as a structural and functional elements. As they are basically just a model of the DNA coating, they do not carry genetic information, but they are very well received by cells. They represent a unique composite material with various biomedical applications and promise to overcome the persisting challenges in the field of nanobiotechnology. They can be widely used, for example, in the area of targeted drug delivery, or in applications such as biosensing, cell modulation or bioimaging. Interactions between engineered DNs and living cells have not yet been sufficiently explored and described. This analysis should help with that as well.