Unravelling novel regulatory mechanisms in the human Na+/H+ antiporter NHA2 (14.11. 2022)
Sodium/proton antiporters are membrane proteins found in all living cells from bacteria to humans and play an important role in regulating intracellular pH and cation concentrations. Among these types of transporters belong also the NHA2 antiporter, whose activity affects a number of physiological functions, e.g. insulin secretion, sodium reabsorption in the kidneys or sperm motility. The NHA2 antiporter transports Na+ or Li+ cations across the membrane in exchange for H+ and its activity is specifically inhibited by phloretin. The properties and functions of all proteins result from their primary structure, i.e. from the sequence of amino acids from which the protein consists. In the family of Na+/H+ antiporters, NHA2 has a unique structure. It consists of 537 amino acids with 14 transmembrane domains and an unique hydrophilic N-terminus long 82 amino acid residues, whose structure and function have not yet been studied. Our new results published in the Protein Science journal revealed several new structural elements important for the NHA2’s function, including the unravelling a new regulatory role of the hydrophilic N-terminus.
We studied the human NHA2 protein and its mutated variants using its expression in a model eukaryotic organism, the yeast Saccharomyces cerevisiae, as well as using bioinformatic simulations (in collaboration with the laboratory of Prof. Nir Ben-Tal from Tel-Aviv University). We newly identified several amino acid residues important for antiporter selectivity (recognition and transport of Na+ and Li+ cations) and for transport of protons. Furthermore, we determined the place in the protein structure where the phloretin inhibitor binds. We also revealed that the unique hydrophilic N-terminal part of the protein has an important (autoinhibitory) role in regulating the transport activity of NHA2, because truncations of the first 50 - 70 residues of the N-terminus doubled the transport activity of the antiporter. Our results also show that the new expression system for NHA2 in yeast cells can be useful for a rapid screening of SNP´s effects on NHA2 activity, and/or to test new compounds influencing the function of NHA2, similarly as phloretin.
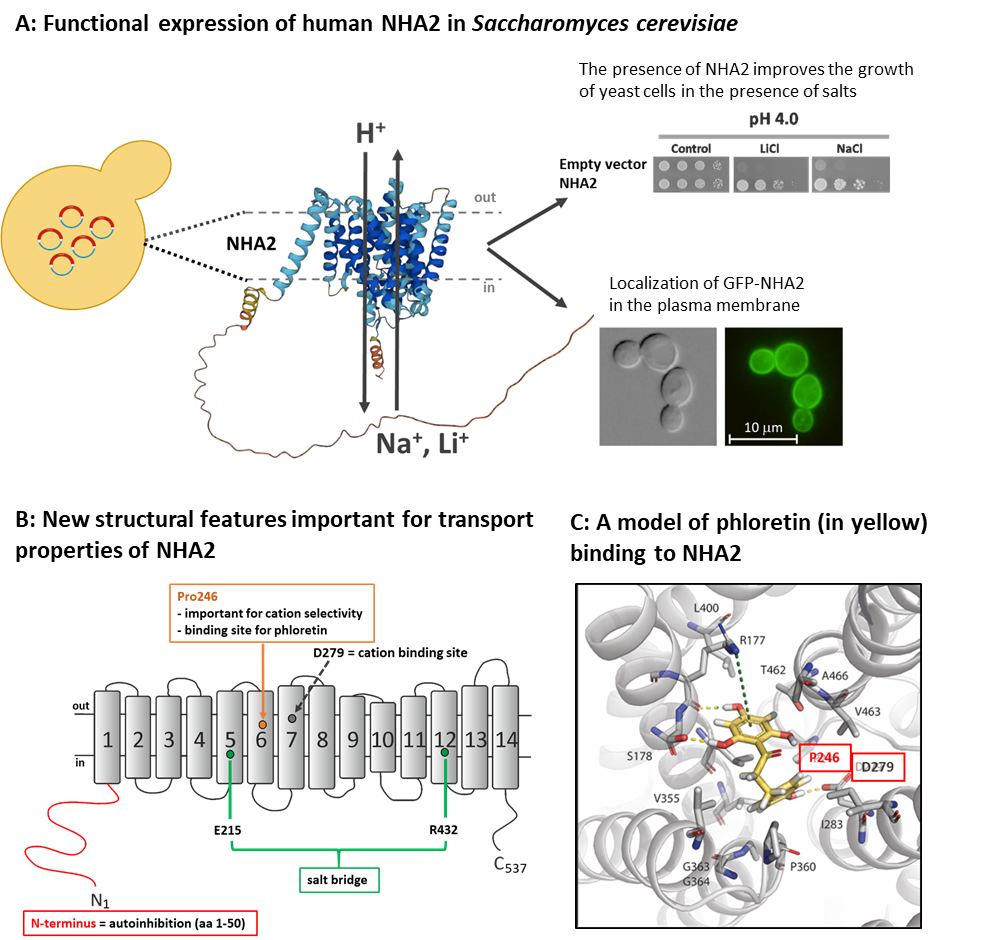
Velazquez D., Prusa V., Masrati G., Yariv E., Sychrova H., Ben-Tal N. and Zimmermannova O. (2022): Allosteric links between the hydrophilic N-terminus and transmembrane core of human Na+/H+ antiporter NHA2. Protein Sci: e4460. IF = 6.993 DOI
NEW PUBLICATION
Thiazolidinediones (TZDs) belong to the category of antidiabetic drugs that increase insulin sensitivity. However, the first generation of TZDs show several side effects, including increased formation of large fat cells (adipocytes) in the bone marrow associated with a higher risk of fractures and bone loss. These side effects are thought to be caused by the strong binding of TZDs to the nuclear receptor PPARγ. In a new study published in the journal Molecular Metabolism, we investigated how administration of MSDC-0602K, a new TZD analog with lower affinity for PPARγ receptors, affects bone metabolism.
We found that administration of MSDC-0602K to obese mice for 8 weeks resulted in significantly better bone microstructure and bone strength, along with an increased proportion of smaller adipocytes in the bone marrow, compared to the original TZDs. We also investigated the effects of MSDC-0602K at the molecular level using primary cell cultures. Bone marrow mesenchymal stem cells from mice treated with MSDC-0602K differentiated to an increased extent into the form of osteoblasts (bone cells), did not undergo as much aging and showed increased cellular metabolism of glutamine, which is important for bone formation (osteogenesis).
Taken together our findings suggests that the new TZD analog could increase insulin sensitivity with less adverse effects on bone quality and mesenchymal stem cell metabolism compared to the original TZDs. Thus, MSDC-0602K could replace older antidiabetic drugs in the treatment of metabolic and bone diseases.
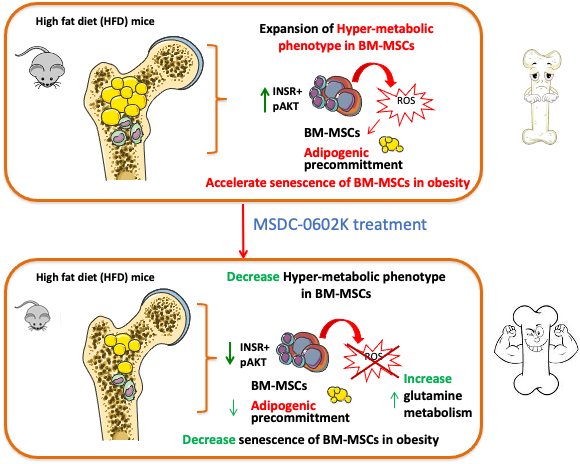
Benova, A., M. Ferencakova, K. Bardova, J. Funda, J. Prochazka, F. Spoutil, T. Cajka, M. Dzubanova, T. Balcaen, G. Kerckhofs, W. Willekens, G. H. van Lenthe, G. Alquicer, A. Pecinova, T. Mracek, O. Horakova, M. Rossmeisl, J. Kopecky and M. Tencerova (2022). "Novel thiazolidinedione analog reduces a negative impact on bone and mesenchymal stem cell properties in obese mice compared to classical thiazolidinediones." Mol Metab 65: 101598. IF = 8.568 DOI
The structure of steroids fundamentally affects their effect at the N-methyl-D-aspartate receptors (20.10. 2022)
NEW PUBLICATION
N-methyl-D-aspartate receptors (NMDARs) are proteins involved in the regulation of many processes in the mammalian brain. They play a key role in signal transmission between nerve cells, and even a small disturbance in the NMDAR function may have sever pathophysiological effects. A reduced function of NMDARs is associated with neuropsychiatric disorders such as mental retardation, reduced intellect, schizophrenia, autism spectrum disorders, epilepsy, or motor disorders. The insufficient NMDAR function can be compensated for by numerous compounds, including neurosteroids. However, the mechanism by which neurosteroids potentiate NMDARs is not well understood.
In a study published in the British Journal of Pharmacology, we investigated the effect of newly synthesized synthetic analogues of endogenous neurosteroid pregnanolone sulfate on NMDARs. We demonstrated that analogues with short aliphatic chains, such as pregnanolone carboxylate (PA-Car), inhibit NMDAR responses, whereas analogues with longer aliphatic chains, such as epipregnanolone butyrate (EPA-But), potentiate NMDAR responses. Combining electrophysiology, molecular biology, and computational modelling, we identified the binding site for EPA-But at the transmembrane domain of NMDAR and suggested the mechanism by which EPA-But enhance the NMDAR function.

(A) Left, structure of pregnanolone carboxylate (PA-Car) and epipregnanolone butyrate (EPA-But). Right, graph shows the average effect of PA-Car and EPA-But at NMDAR. (B) Location of the EPA-But binding site at the transmembrane domain of NMDAR. Amino-acid residues that are involved in the interaction with EPA-But molecule (green) are highlighted in red.
Kysilov B, Hrcka Krausova B, Vyklicky V, Smejkalova T, Korinek M, Horak M, Chodounska H, Kudova E, Cerny J, Vyklicky L: Pregnane-based steroids are novel positive NMDA receptor modulators that may compensate for the effect of loss-of-function disease-associated GRIN mutations. British Journal of Pharmacology, (2022) 179:15, 3970–3990, IF = 9.473, DOI
Ion channel TRPC5 as a target for treating neuropathic pain (19.10. 2022)
Despite great advances in medicine, treatment options for pain states associated with diabetes or chemotherapy-induced neuropathy are still limited. These types of neuropathies are accompanied by cold-induced pain that is very difficult to manage in clinical practice. In fact, the only drug that has successfully undergone clinical trials and demonstrated efficacy for this type of pain so far is duloxetine. This drug is superior to other antidepressants with a similar mechanism of action at managing painful neuropathies, but it is not known why. Interestingly, it has recently been shown that the cold-sensitive TRPC5 ion channel is expressed in human sensory neurons and that inhibition of its activity relieves persistent pain, including neuropathic cold pain.
In our study we asked whether the TRPC5 channel is modulated by duloxetine and may contribute to its analgesic effect. Our electrophysiological measurements showed that TRPC5 channel activity is strongly suppressed by duloxetine. We performed molecular docking and molecular dynamic simulations that identified a potential biding site for duloxetine. Subsequent point mutagenesis validated that the duloxetine molecule resides in a well-known biding pocket on the intracellular side of the TRPC5 transmembrane domain. Slight manipulation of the shape and electrostatic of the binding pocket environment (replacing the amino acid glutamate 418 with alanine) caused a complete loss of the duloxetine effect on voltage-evoked TRPC5 activity. Our results suggest that TRPC5 is a previously unrecognised target for a commonly used, highly effective drug against severe forms of pain. Furthermore, the finding that this TRPC5 inhibitor is widely used and well tolerated provides a scaffold for new pain treatment strategies.

Zimova L, Ptakova A, Mitro M, Krusek J,Vlachova V: Activity dependent inhibition of TRPC1/4/5 channels by duloxetine involves voltage sensor-like domain, Biomedicine & Pharmacotherapy. Roč. 152, August 2022, 113262, IF = 7,419, DOI
New lipid classes in human breastmilk (29.7. 2022)
Breastfeeding is the best way of nutrition for a newborn baby. Mother's milk provides the ideal cocktail of nutrients and bioactive components for rapid growth and development in this early period of human life. Milk composition can be affected by several factors such as maternal dietary habits, mode of delivery, and pregnancy length. An essential question for neonatologists is just the optimal composition of breastmilk for premature babies.
In our study, we investigated the impact of preterm birth and a cesarean section on the quality of the first form of milk – colostrum. Analysis of the samples obtained from mothers at the Institute for the Care of Mother and Child in Prague showed that both the mode and term of delivery slightly negatively affected the composition of colostrum. In these cases, the mammary gland of mothers did not have enough time to initiate fully-fledged milk production. Still, this negative effect disappears in time, and mature milk composition resembles the milk of mothers who delivered spontaneously in term.
Moreover, we discovered a peculiar class of lipids called triacylglycerol estolides in all milk samples, which serves as a reservoir of the antiinflammatory lipids. We found out that the milk enzyme carboxyl ester lipase can digest these complex molecules and release substances with beneficial effects on the health of newborns.
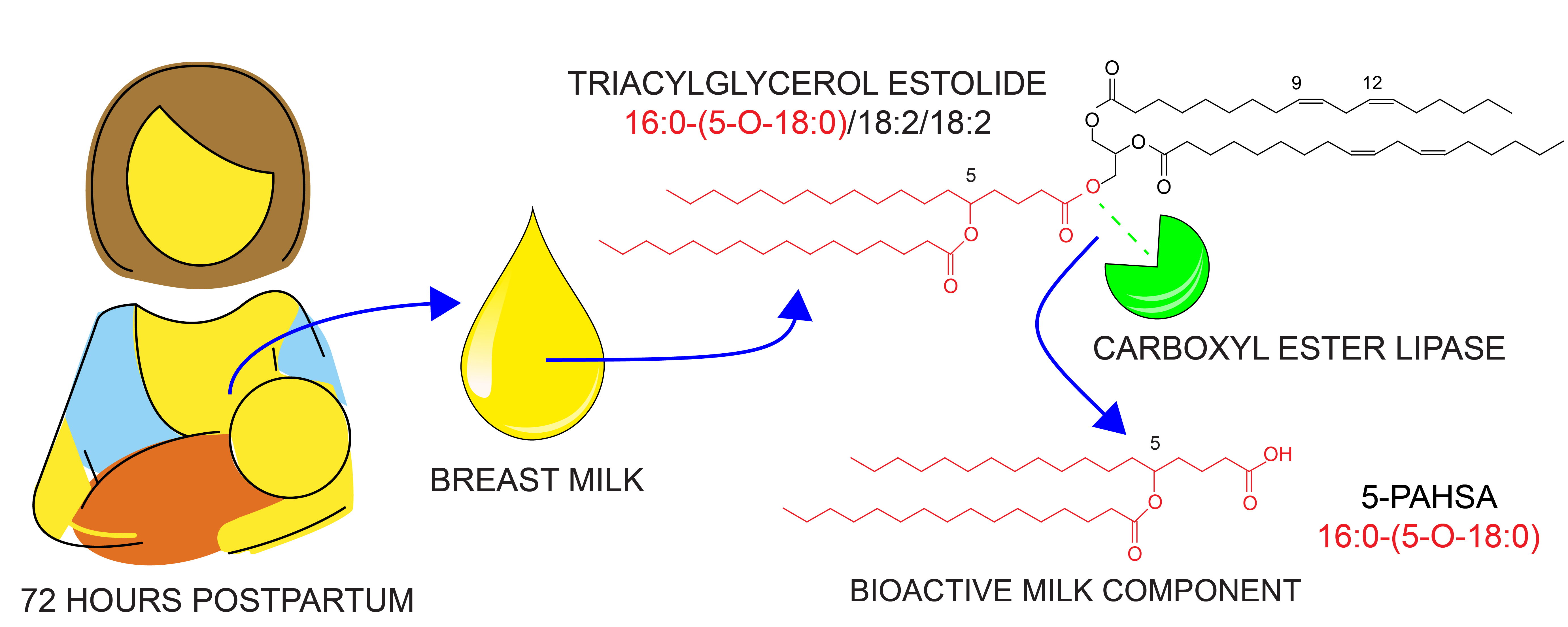
Brejchová K, Palůchová V, Březinová M, Čajka T, Balas L, Durand T, Křížová M, Straňák Z, Kuda O: Triacylglycerols containing branched palmitic acid ester of hydroxystearic acid (PAHSA) are present in the breast milk and hydrolyzed by carboxyl ester lipase, Food Chemistry. 2022; 388(Sep 15)); 132983, IF = 9.231 DOI
Load next





