Design, synthesis and biological profiling of novel fused deazapurine nucleosides
PI: Michal Hocek, co-PI: Helena Mertlíková-Kaiserová
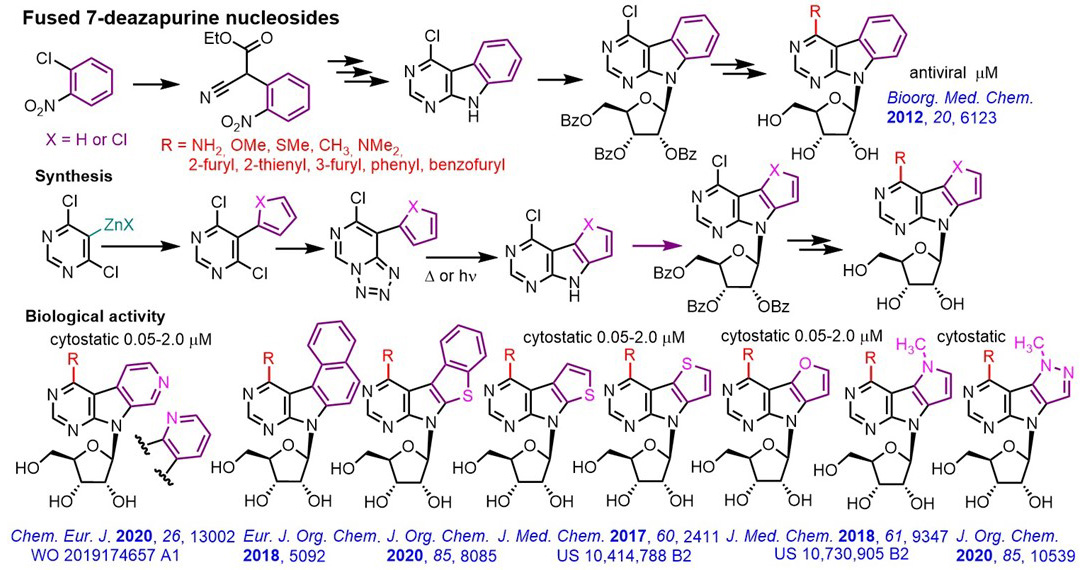
The main goal of this project is the design and synthesis of a new generation of novel nucleoside derived from benzo- or hetero-fused 7-deazapurines and profiling of their biological activity. The synthesis of the fused nucleobases was either based on multistep heterocyclization or on cross-coupling, azidation and C-H activation, and the follow up glycosylation and modification of the nucleobase provided the desired modified fused nucleoside. The benzo-fused (pyrimidoindole) nucleosides were non-cytotoxic but still exerted micromolar activities against some RNA viruses (HCV, dengue). On the other hand, the novel thieno-fused 7-deazapurine nucleosides displayed cytostatic activities in submicromolar to nanomolar concentrations. Therefore, we decided to systematically prepare and study diverse hetero-fused deazapurine nucleosides. So far, we prepared furo-, pyrrolo-, pyrazolo-, different isomers of pyrido-, naphtho-, benzothiazolo-fused analogues, some of which are also nanomolar cytostatics. The mechanism of action involved activation by phosphorylation and incorporation to DNA causing DNA damage and apoptosis. The active compounds were patented and selected examples are undergoing preclinical ADMETox and in vivo studies.




