Thanks to his new discovery, Pavel Hobza stands a great chance at rewriting the textbooks of physical chemistry
Pavel Hobza from the Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences and his team first contributed to the rewriting of textbooks twenty years ago. This was when they discovered and described the so-called improper hydrogen bond. Together with colleagues from the Institute of Physical Chemistry of Jaroslav Heyrovský and the VSB – Technical University in Ostrava, he has made yet another discovery. This time it has the potential to simplify the previously adopted definition of the hydrogen bond and to force chemistry students and researchers to rethink their ideas about the substances they study.
In a previous article, Pavel Hobza's team described the improper hydrogen bond X–H…Y. This is not manifested by an expected red shift (towards lower frequencies) in the vibrational frequency of the X–H bond, which is involved in the hydrogen bond, but, on the contrary, by a blue shift (towards higher frequencies). In a recent study published in the Journal of the American Chemical Society, the authors propose a new refinement and simplification of the definition of hydrogen bonding: In addition to the protonic bond, the definition should newly include the hydridic hydrogen bond.
"The current definition of the hydrogen bond is based on our discovery of the improper hydrogen bond, characterized by a blue rather than the expected red shift in the vibrational frequency of the X–H bond. Our recent studies, however, go even further. They have shown that hydrogen bonds form even in the case of hydridic and not only protonic hydrogen. We therefore propose to modify the current definition of the hydrogen bond to include all types of bonds," explains Professor Pavel Hobza.

Water—with its familiar formula H2O—is a very simple molecule made up of one oxygen atom and two hydrogen atoms, hydrogen being the lightest of all the existing elements. That water flows from the tap as a liquid and that it boils at a temperature of 100°C is all down to the existence of the so-called hydrogen bond. It is formed between one of the hydrogen atoms of one water molecule and the oxygen atom of another. Thanks to such non-covalent interactions, the double helices of DNA hold together, and just as importantly, these interactions also play key roles in the structure and functioning of all proteins, including enzymes. Hydrogen bonding therefore plays an utterly essential and indispensable role in most chemical and virtually all biochemical processes on the planet.
Most elements in the periodic table have a lower electronegativity—the ability to attract electrons—than hydrogen. Only a few elements (e.g., carbon, nitrogen, oxygen and halogens) have higher electronegativity. In the water molecule, oxygen attracts the electrons of the hydrogen atoms, which then become partially positively charged. If a molecule containing an element with surplus of electrons such as oxygen or nitrogen, is found near a positively charged hydrogen, a protonic hydrogen bond is formed. This weakens and lengthens the bond between the hydrogen and the more electronegative atom. Such a lengthening causes a decrease in the vibration frequency of this bond – a so-called red shift, which can be measured by infrared spectrometry. The chemical bond thus acts like a string: When it is lengthened, the frequency of its vibration decreases and, conversely, the wavelength increases towards the red part of the spectrum. A similar phenomenon is known from playing the guitar, where the pitch of the sound is controlled by changing the length of strings on the fingerboard.

In some cases, however, the bond between hydrogen and a more electronegative element strengthened, which is manifested by an increase in its vibrational frequency – a so-called blue shift. Such bonds are referred to as improper hydrogen bonds, which are the original discovery of Pavel Hobza.
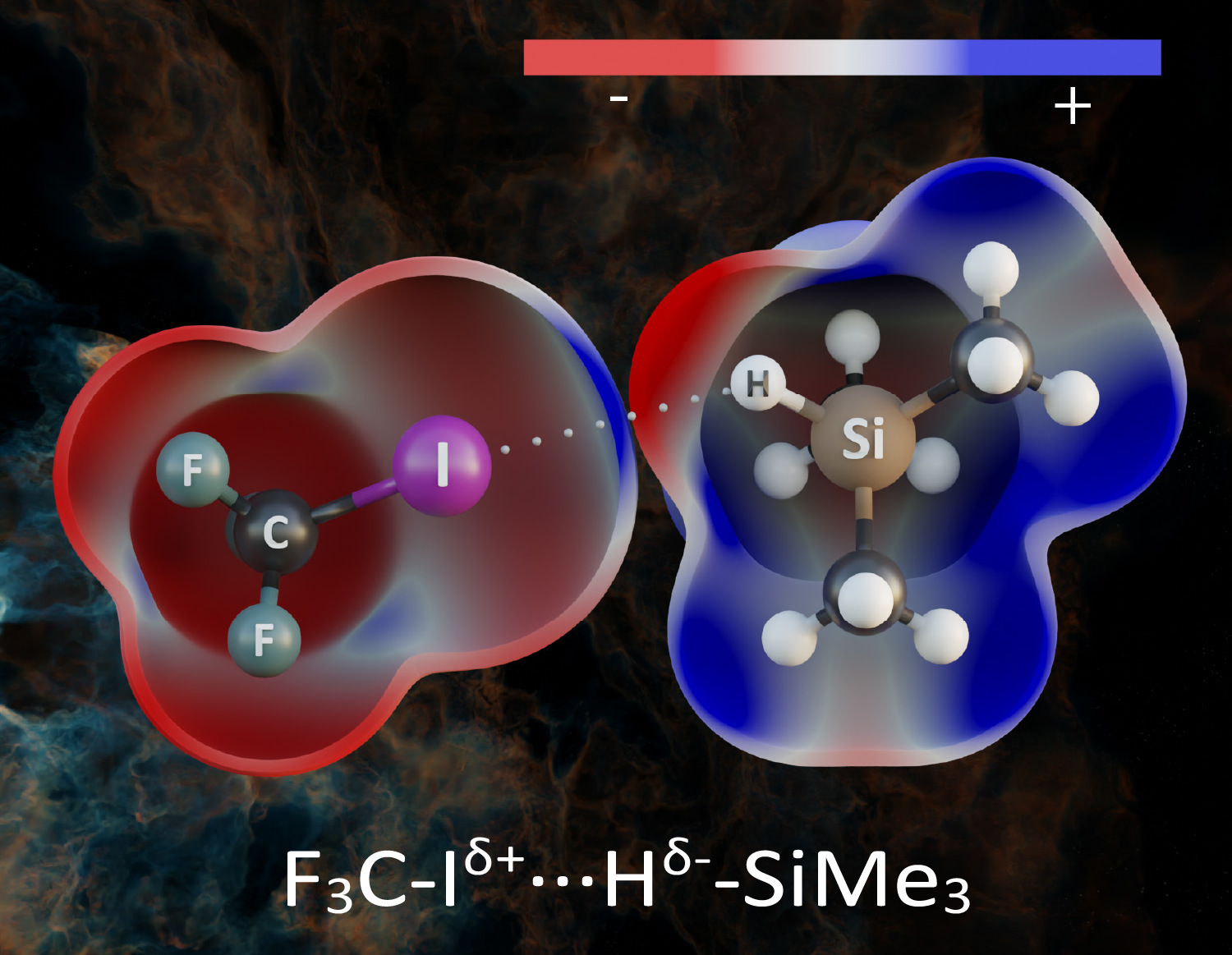
If, however, a hydrogen atom binds to an element with lower electronegativity, the hydrogen becomes negatively charged. Pavel Hobza and his colleagues recently investigated the organosilicon compound trimethylsilane, (CH3)3SiH, where the hydrogen atom is bound to a less electronegative silicon atom and thus carries a negative charge, in complexes with different electron deficient molecules. The hydrogen bond which thus arises is termed a hydridic hydrogen bond. Using computational methods, they found that under certain circumstances, the covalent bond between silicon and hydrogen weakens and lengthens, while its vibrational frequency decreases. A red shift therefore takes place, like it does with a protonic hydrogen bond. The authors of the study are the first to have experimentally demonstrated this red shift in the hydridic hydrogen bond. To this end, they employed low-temperature infrared spectrometry. They were thus able to prove that the hydridic hydrogen bond manifests itself quite analogously to a protonic hydrogen bond.
With this discovery, the time has come to revise the existing IUPAC definition of hydrogen bonding. However, the question remains whether it is necessary to introduce a completely new definition for this type of bonding, or whether to modify the current one. The authors consider the second option more appropriate and, at the end of their publication in the Journal of the American Chemical Society, propose a new version of the definition which includes both types of hydrogen bond: protonic and hydridic.
Original article
Civiš, S.; Lamanec, M.; Špirko, V.; Kubišta, J.; Špeťko, M.; Hobza, P. Hydrogen Bonding with Hydridic Hydrogen–Experimental Low-Temperature IR and Computational Study: Is a Revised Definition of Hydrogen Bonding Appropriate? J. Am. Chem. Soc. 2023, 145, 8550-8559. https://doi.org/10.1021/jacs.3c00802