Kalendář akcí
Zobrazit vše
Naši vědci v médiích – výběr z článků, rozhovorů, reportáží
Publikace
Všechny publikace
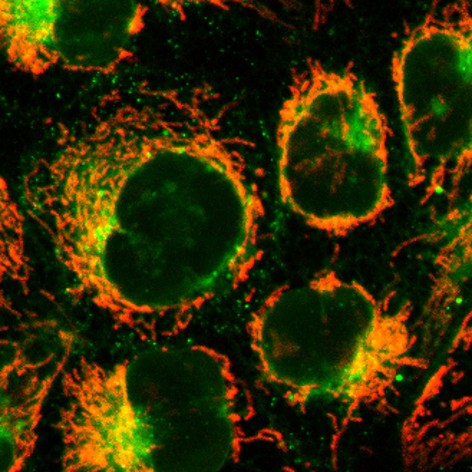
Triazinium Ligation: Bioorthogonal Reaction of N1-Alkyl 1,2,4-Triazinium Salts
Angewandte Chemie International Edition 2023: Early View
The development of reagents that can selectively react in complex biological media is an important challenge. Here we show that N1-alkylation of 1,2,4-triazines yields the corresponding triazinium salts, which are three orders of magnitude more reactive in reactions with strained alkynes than the parent 1,2,4-triazines. This powerful bioorthogonal ligation enables efficient modification of peptides and proteins. The positively charged N1-alkyl triazinium salts exhibit favorable cell permeability, which makes them superior for intracellular fluorescent labeling applications when compared to analogous 1,2,4,5-tetrazines. Due to their high reactivity, stability, synthetic accessibility and improved water solubility, the new ionic heterodienes represent a valuable addition to the repertoire of existing modern bioorthogonal reagents.
Strong CO2 adsorption in narrow-pore ADOR zeolites: A combined experimental and computational study on IPC-12 and related structures
Journal of CO2 Utilization 74: 102548 (2023)
Structure of monkeypox virus poxin: implications for drug design
Archives of Virology 168: 192 (2023)
Generic Platform for the Multiplexed Targeted Electrochemical Detection of Osteoporosis-Associated Single Nucleotide Polymorphisms Using Recombinase Polymerase Solid-Phase Primer Elongation and Ferrocene-Modified Nucleoside Triphosphates
ACS Central Science 2023: Early View