More than one kind of white! Make your own spectroscope and reveal what’s the difference between light coming from various light sources. Compare light sources in your home (Is there a difference between the light in your bathroom and the light above your kitchen countertop?) or the light emitted by different lamps in your street. What makes them look different?
FZU POCKET SPECTROSCOPE – FREE DOWNLOAD HERE:
The visible light spectrum
Light can be thought of as electromagnetic waves spreading through space. Light is actually a narrow interval of wavelengths visible to us. The other types of electromagnetic waves include radio waves or ultraviolet radiation.
Light of different wavelengths is detected by the human eye as different colours. The human eye often detects light of multiple wavelengths (colours) at the same time, giving an overall colour impression that combines several colours – for example mixing blue and yellow light gets us light that is seemingly green. Similarly, light that we perceive as white is actually a combination of many different colours. If we disperse light into all constituent colours we get something known as the light spectrum.
To decompose light, we can use a simple device such as our pocket spectrometer. If we use this device to take a look at different light sources, we can explore the composition of light. Try different types of light sources even if, at first sight, they seem to emit similar light.
Basic Types of Spectrum
Continuous spectrum
If you target your spectroscope at a candle flame or a classic light bulb, you will see all colours ranging from red to violet. It’s because the light is created as a result of strong heating of air or – of light bulb filament to several hundreds of degrees Celsius. Each body emits electromagnetic radiation of all wavelengths, with its intensity rising proportionally to rising temperature. In addition, the maximum radiation shifts towards shorter wavelengths. Therefore, everyday objects around us emit the most light in the invisible infrared region. Only very hot bodies emit enough light for the human eye to detect it.
Emission line spectrum
A gas-filled fluorescent lamp can emit pleasant yellow light similar to that of the candle. Yet its spectrum looks quite different. It typically contains only several colours that we can see in a spectroscope as colour lines on a dark background. That’s why they’re called emission spectral lines and their spectrum is called a line spectrum.
This is because a fluorescent lamp generates light in a totally different way than in the case of the continuous spectrum. By touching the lamp we can confirm that this way has, indeed, nothing to do with the heating up of gas. In fact, light releases also when electrons move between different levels within atomic electron shells. When an electron moves to a lower level, it loses its energy and releases it in the form of light. The energy difference between the levels cannot be arbitrary. The electron shell structure is typical for every chemical element. As a result, every element has its own unique line spectrum structure.
By turning on a fluorescent lamp we give energy to electrons in the gas electron shells. This energy moves them to higher energy levels. Electrons have a tendency to return to more stable lower levels, emitting light. As there is a huge number of atoms, they emit enough light to find practical application. In fact, the situation is even more complex because atoms often emit also invisible electromagnetic radiation instead of light, and it’s only the intransparent surface luminophore layer of the lamp that absorbs them, and re-emits them in the visible region. But this doesn’t change anything about the spectrum’s linear nature.
Absorption line spectrum
There is also the opposite phenomenon whereby an electron absorbs the light and jumps up to a higher level within the electron shell. If, for example, continuous thermal radiation goes through a relatively colder gas, some colours are not present in the spectrum, which results in the dark lines – absorption spectral lines and the absorption spectrum. Every chemical element shows a typical line structure, whether they are emission or absorption lines. A typical example of the absorption spectrum is the Sun’s spectrum (Don’t point your spectroscope directly at the Sun, just aim at any direction in the sky during the day!) where the continuous region is emitted by the depths of our mother star, while the dark lines come from the outer layers of the Sun’s atmosphere where the temperature is “mere” thousands of degrees Celsius. To observe them, however, we need spectroscopes with a very narrow slit. We’re going to discuss how the width of the slit influences what the spectrum looks like in the next paragraph.
How does the spectroscope work?
Our pocket spectroscope is a paper box. It contains only several basic elements. There is a passage through which light comes inside; a spot where the light disperses; and a point where we observe the dispersed light. In other words, the box contains a slit; a dispersing element; and an observation window.
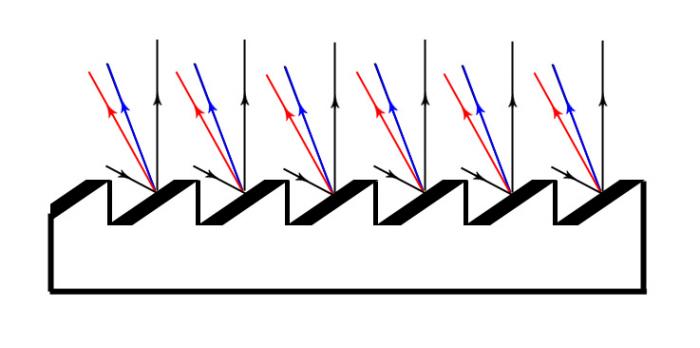
A dispersing element is a generic term for elements capable of dispersing light. A classic example is a glass prism. But more frequently, scientists use an optical grid with very subtle parallel incisions on its surface, which pass or reflect light. In our pocket spectroscope, we also use an optical grid, which is a reflective piece cut out from an old CD, with data burned onto it; it’s the burned data that give us the required structure.
If we observe light reflections from the CD outside the paper spectrometer, we can see different rainbow colours but never any spectral lines. The explanation is that the CD is hit by rays from different directions, and, as a result, we observe a lot of different spectra laid randomly over one another. If we wish to see spectral lines, we must let the light come in from a narrow cut-out of all the directions. Therefore, we observe the sources through a slit. Spectra lines are actually the image of the slot that reflects in each colour under a slightly different angle from the optical grid. The narrower the slit, the sharper the spectral lines. However, as less light will pass through the slit, the spectrum will become weaker. Then all we need is a fair compromise. The best thing to do is to cut out a strip of paper, which should be as narrow as possible; cutting it with a knife is not desirable.
How are the spectra used by scientists?
Have you tried your pocket spectroscope yet? Were you also surprised how many types of spectra there are around us? As we mentioned earlier, the spectrum appearance is derived from how the light came into being and what was happening to it as it was spreading through its environment. Advanced high-sensitivity and high-definition spectrometers can reveal striking findings to researchers.
If we let the light go through or reflect from a sample, part of such light will be absorbed by the atoms of the substance, creating typical absorption lines that define a chemical element or a compound. Therefore spectroscopy is one of the basic tools for chemical composition analysis, for medicine (such as toxic substances in blood samples) and the food processing industry. Apart from optical spectrometry, there are other types of spectroscopes such as infrared spectroscopes or spectroscopes based on measuring the energy of flying electrons instead of photons.
A scientific discipline that doesn’t have many other tools apart from light and, in generic terms, electromagnetic radiation, is astrophysics. Spectroscopy has played an indispensable role in the exploration of the universe. In addition, spectroscopy allows scientists to study physical conditions – specific spectral lines can only occur at certain temperatures and pressure; the shift of lines within the spectrum (Doppler’s effect) on the other hand helps them track the motion of celestial bodies.