 Recent methodology and hardware development significantly increases resolution and selectivity of solid-state NMR experiments that can now provide detailed information about structure and dynamics of wide range of materials. It has to be mentioned that quite recently three dimensional structures of several uniformly labeled proteins have been successfully determined by solid-state NMR. That is why we believe that nowadays it is not too bold to affirm that solid-state NMR spectroscopy is almost comparable with well-established X-ray diffraction techniques. Consequently our research interest is focused to develop and apply new solid-state NMR techniques to measure heteronuclear dipolar couplings in materials at natural isotopic abundance in order to precisely describe structure and dynamics of highly organized systems (microcrystalline substances, polymers, nanocomposites and geopolymers).
Recent methodology and hardware development significantly increases resolution and selectivity of solid-state NMR experiments that can now provide detailed information about structure and dynamics of wide range of materials. It has to be mentioned that quite recently three dimensional structures of several uniformly labeled proteins have been successfully determined by solid-state NMR. That is why we believe that nowadays it is not too bold to affirm that solid-state NMR spectroscopy is almost comparable with well-established X-ray diffraction techniques. Consequently our research interest is focused to develop and apply new solid-state NMR techniques to measure heteronuclear dipolar couplings in materials at natural isotopic abundance in order to precisely describe structure and dynamics of highly organized systems (microcrystalline substances, polymers, nanocomposites and geopolymers).
events
« actual »
« realized »
Laboratory ssNMR
since: 1987new equipment 2003
actual system:
Bruker AVANCE 500 US/WB
 head of the laboratory:
head of the laboratory:Ing. Jiří Brus, PhD
contact: brus@imc.cas.cz
Research topic
 Recent methodology and hardware development significantly increases resolution and selectivity of solid-state NMR experiments that can now provide detailed information about structure and dynamics of wide range of materials. It has to be mentioned that quite recently three dimensional structures of several uniformly labeled proteins have been successfully determined by solid-state NMR. That is why we believe that nowadays it is not too bold to affirm that solid-state NMR spectroscopy is almost comparable with well-established X-ray diffraction techniques. Consequently our research interest is focused to develop and apply new solid-state NMR techniques to measure heteronuclear dipolar couplings in materials at natural isotopic abundance in order to precisely describe structure and dynamics of highly organized systems (microcrystalline substances, polymers, nanocomposites and geopolymers).
Recent methodology and hardware development significantly increases resolution and selectivity of solid-state NMR experiments that can now provide detailed information about structure and dynamics of wide range of materials. It has to be mentioned that quite recently three dimensional structures of several uniformly labeled proteins have been successfully determined by solid-state NMR. That is why we believe that nowadays it is not too bold to affirm that solid-state NMR spectroscopy is almost comparable with well-established X-ray diffraction techniques. Consequently our research interest is focused to develop and apply new solid-state NMR techniques to measure heteronuclear dipolar couplings in materials at natural isotopic abundance in order to precisely describe structure and dynamics of highly organized systems (microcrystalline substances, polymers, nanocomposites and geopolymers).
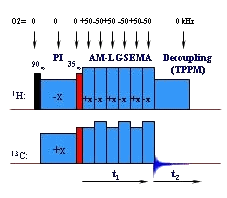
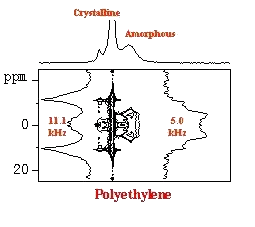 The key aim is the development of the techniques for determination of sufficient number of long-range (1H-13C) distance restraints to reconstruct structure of microcrystalline pharmaceutically active substances. The extension of these techniques to nuclei with half integer spin (27Al) is motivated to probe structural motifs in amorphous alumino-slicates (geopolymers). The goal is to discover relationships between structure and material properties. Finally the development of site-specific techniques to measure motionally averaged one-bond 1H-13C dipolar couplings selectively according to relaxation behavior of polymer segments will open new way how to analyze amplitudes of segmental motions. Investigation of relations between dynamics and material properties affecting applicability of polymers is non-negligible part of our investigation.
The key aim is the development of the techniques for determination of sufficient number of long-range (1H-13C) distance restraints to reconstruct structure of microcrystalline pharmaceutically active substances. The extension of these techniques to nuclei with half integer spin (27Al) is motivated to probe structural motifs in amorphous alumino-slicates (geopolymers). The goal is to discover relationships between structure and material properties. Finally the development of site-specific techniques to measure motionally averaged one-bond 1H-13C dipolar couplings selectively according to relaxation behavior of polymer segments will open new way how to analyze amplitudes of segmental motions. Investigation of relations between dynamics and material properties affecting applicability of polymers is non-negligible part of our investigation.
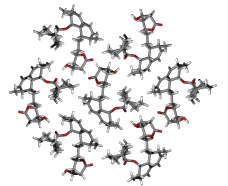

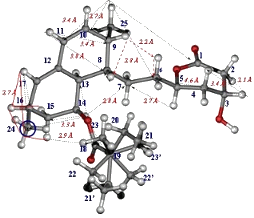
Determination of complete three-dimensional structure of medium size organic molecules in solid microcrystalline state at natural isotopic abundance is the main task for pharmaceutical research. Majority of the active substances do not usually form single crystals suitable for x-ray diffraction studies.
(Pharmaceutical application, solid form identification - polymorphs, solvates, hydrates, isomorphic crystal forms, static vs. dynamic disorder, hydrogen bonding)
 There are a variety of different applications of solid-state NMR spectroscopy that are relevant to pharmaceutical research. Some of them include analysis of solid forms (polymorphs, solvates), hydrogen bonding and crystal packing and/or solid-solid interactions (phase transformations, activation energies of molecular motions). The study of polymorphism appears to be one of the most common applications of solid-state NMR spectroscopy for pharmaceutical compounds. During past ten years predominantly
13
C CP/MAS NMR spectroscopy was used to study and distinguish different forms of various drugs simply based on differences in isotropic chemical shift. Due to the increasing intensity of static magnetic field and decoupling fields resolution of
13
C CP/MAS NMR spectra has been recently significantly enhanced.
There are a variety of different applications of solid-state NMR spectroscopy that are relevant to pharmaceutical research. Some of them include analysis of solid forms (polymorphs, solvates), hydrogen bonding and crystal packing and/or solid-solid interactions (phase transformations, activation energies of molecular motions). The study of polymorphism appears to be one of the most common applications of solid-state NMR spectroscopy for pharmaceutical compounds. During past ten years predominantly
13
C CP/MAS NMR spectroscopy was used to study and distinguish different forms of various drugs simply based on differences in isotropic chemical shift. Due to the increasing intensity of static magnetic field and decoupling fields resolution of
13
C CP/MAS NMR spectra has been recently significantly enhanced.
This leads also to resolution of nonequivalent molecules in the asymmetric unit of the crystal. This fact together with newly developed techniques for unambiguous signal assignment makes it possible to study very fine changes in the conformational structure of molecules during drug processing. Relatively low detection limit ca 2-5 wt % indicates possibility of analysis complex mixtures or dosage products. Solid-state NMR spectroscopy is also used to analyze drugs in tablets to identify drug-excipient interactions (eg. formation or breaking of hydrogen bonds, restriction of segmental mobility, etc).
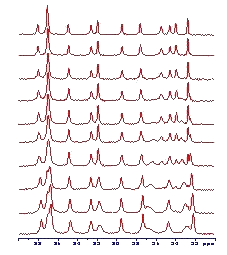
(Molecular dynamics and conformation in polypetides. Effect of hydration on segmental dynamics and elastic properties)
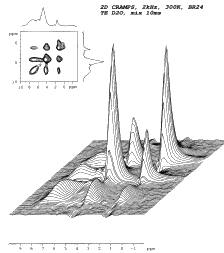

In recent past we dealt with determination of structure, morphology and dynamics of several synthetic polymer systems (blends of poly(ethylene oxide) and polycarbonate, hybrid siloxane networks, siloxanes based on hyperbranched polymers) as well as natural substances like coals or humic acids. Gradually we focused our attention on the determination of relationships between hydration degree and conformation and conformational dynamics of peptides and polypeptides which mimics extracellular elastine-like matrix proteins. We studied the overall changes of morphology of copolymer systems containing peptide block and block of poly(ethylene oxide), which can affect mechanical and bioactive properties of these materials used in the field of tissue engineering and temporary scaffold for the cell transplantation.
In order to investigate the interaction of short hydrophobic and hydrophilic polypeptides systematically, a series of model sequences were studied. The main technique used for the conformational study was solid-state NMR spectroscopy in combination with ab-initio, molecular mechanics and molecular dynamics calculations of molecular properties and geometry.






