The study of structure and function of 14-3-3 proteins and their complexes
Our research team has been studying the 14-3-3 proteins which are highly conserved regulatory molecules found in all eukaryotes. 14-3-3 proteins have the ability of binding the functionally different signal proteins, including kinases, fosfatases and transmembrane receptors by changing their function. Through the functional modulation of a wide range of binding partners, 14-3-3 proteins are involved in many processes, including cell cycle regulation, metabolism control, apoptosis, and control of gene transcription. More than 300 proteins have been described as binding partners till now. We employ both biophysical (fluorescence spectroscopy, analytical ultracentrifugation, SAXS, mass spectrometry, isothermal titration calorimetry, X-ray crystallography, protein structure modeling, etc.) and biochemical (recombinant protein expression, site-directed mutagenesis, enzyme kinetics) approaches to understand the details of how the activity and function of protein-protein complexes are regulated.
Research Projects
The 14-3-3 proteins are a family of regulatory molecules, which specifically bind to phosphoserine (or phosphothreonine)- containing motifs (pSer/pThr) in a sequence-specific manner. Through these binding reactions, the 14-3-3 proteins play key regulatory roles in signal transduction, cell cycle control, metabolism control and apoptosis. More than 200 14-3-3 binding partners have been reported so far and some of them play prominent roles in cancer development (e.g. transcription factors p53 and FOXO), neurodegeneration (e.g. Tau protein, ASK1 kinase), cardiovascular diseases (e.g. RGS proteins, phosducin) or inflammation (e.g. NFkB, ASK1 kinase). However, the detailed mechanisms of the14-3-3 protein-mediated regulations are mostly elusive, mainly due to the lack of structural data.
Main goal of our research is a mechanistic understanding of the 14-3-3 protein function in the regulation of selected 14-3-3 protein binding partners. In recent years we have been studying the 14-3-3 protein-mediated regulation of forkhead transcription factor FOXO4, tyrosine hydroxylase, or regulator of G-protein signaling RGS3. Our current projects are focused on regulation of caspase-2, proten kinase CaMKK2, FOXO-DBD, neutral trehalase Nth1, protein kinase ASK1.
Caspase-2
Caspase-2 (C2), a cysteine-dependent and aspartate-specific intracellular protease, has multiple roles in the DNA damage response, cell cycle regulation and tumor suppression. C2 functions as a central coordinator between the cell metabolism and apoptosis and its function is regulated by phosphorylation at several Ser residues. Phosphorylated procaspase-2 (proC2) binds to the 14-3-3 protein and this interaction blocks proC2 activation through an unknown mechanism. To elucidate this regulatory mechanism we propose to: i) identify sites responsible for the 14-3-3 protein binding to proC2, ii) perform biophysical characterization of the 14-3-3:proC2 complex using analytical ultracentrifugation, iii) map the binding interface of the 14-3-3:proC2 complex and perform its structural analysis using hydrogen-deuterium exchange coupled to MS, SAXS and protein crystallography. The proposed research is expected to provide the structural insight into the 14-3-3-dependent regulation of C2. This project is funded by Czech Science Foundation (Project 17-00726S).
Results were published in: Kalabova et. al. (2020) FEBS Journal; Smidova et. al. (2018) FEBS Journal; Kalabova et. al. (2017) BBRC.

SAXS-based structural modeling of the complex between procaspase-2 and 14-3-3 protein (Smidova et. al (2018) FEBS Journal).

Interactions between 14-3-3 and the 14-3-3-binding motifs of caspase-2. (A) Crystal structure of the 14-3-3gamma:pepS139 complex. The 2Fo-Fc electron density map is contoured at 1σ. PDB ID: 6GKF (B) Detailed view of contacts between 14-3-3c and the pepS139 peptide. The caspase-2 residues are labeled in red, and the 14-3-3c residues are labeled in black. (C) Crystal structure of the 14-3-3c:pepS164 complex. The 2Fo-Fc electron density map is contoured at 1σ. PDB ID: 6GKG. (D) Detailed view of contacts between 14-3-3gamma and the pepS164 peptide. The caspase-2 residues re labeled in red, and the 14-3-3c residues are labeled in black (Smidova et. al (2018) FEBS Journal).
FOXO3-DBD
FOXO transcription factors control apoptosis, stress resistance and longevity in mammalian cells. Although the members of the FOXO family act as tumor suppressors in some cell types, emerging evidence suggests that FOXO3 also contributes to tumor stem cell renewal, immune suppression, metastases and chemotherapy resistance in certain cancer types. By a combined in silico / cell biological screening approach several small, drug-like compounds that interact with the DNA-binding domain of FOXO3 and efficiently inhibit its transcriptional activity have been identified. The main aim of this project is to define the structural basis for the interaction between compounds and FOXO3 by NMR spectroscopy, design and develop derivatives with improved properties regarding solubility and affinity, analyze compound-dependent inhibition of target recognition by FOXO3 in vitro and in vivo, study the effects of compounds on FOXO3-induced cancer cell survival in 3D cell culture and in vivo. This project is a collaboration with the group of prof. Michael J. Ausserlechner from Medical University Innsbruck, Innsbruck, Austria, and it is funded by Czech Science Foundation (Project 17-33854L).
Results were published in: Hagenbuchner et.al. (2019) eLife; Psenakova et. al. (2019) Cells

Compounds S9 blocks the DNA binding surface of Forkhead transcription factor FOXO3. The figure shows the structural model of the DNA-binding domain of FOXO3 with bound compound S9 based on data from NMR measurements and docking simulations.

Solution structure of the free FOXO1 DNA binding domain (Psenakova et al. (2019) Cells).
CaMKK2
The Ca2+/calmodulin-dependent protein kinase (CaMK) cascade is involved in the regulation of many physiological and pathophysiological processes. This signaling cascade consists of CaMKI and CaMKIV and their upstream activator CaMK kinase (CaMKK). The activity of CaMKK is inhibited through phosphorylation by PKA in a process involving the binding to the 14-3-3 protein. However, the molecular mechanism of this 14-3-3-mediated inhibition of CaMKK is currently unknown. Anticipated mechanisms include direct inhibition through structural modulation of the catalytic site, blocking of dephosphorylation of inhibitory phosphorylation sites or interference with the binding of Ca2+/calmodulin to CaMKK. Main goal of this project is to elucidate the molecular basis of this regulation by performing functional and structural analysis of interactions between 14-3-3 and CaMKK2. This project is funded by Czech Science Foundation (Project 16-02739S).
Results were published in: Kylarova et. al. (2018) BBA-General Subjects; Psenakova et. al. (2018) BBA-General Subjects.
Molecular basis of the 14-3-3 protein-dependent activation of yeast neutral trehalase Nth1
We deciphered the mechanism by which the 14-3-3 protein regulates the function of yeast neutral trehalase Nth1. Neutral trehalase Nth1 catalyzes the hydrolysis of disaccharide trehalose and represents one of the key enzymes in the energetic metabolism of yeast. We solved the crystal structures of several forms of neutral trehalase Nth1 and deciphered the changes which occur upon the ligand and 14-3-3 protein binding. We showed that the catalytic activity of Nth1 is enabled by the proper 3D configuration of Nth1´s catalytic and calcium-binding domains relative to each other, which stabilizes the flexible part of the active site required for catalysis. This finalized our long-lasting research focused on understanding of the mechanism of yeast neutral trehalase regulation. Solved atomic structure of the 14-3-3 protein complex with neutral trehalase Nth1 shows the ability of the 14-3-3 protein to modulate the structure of a multidomain enzyme and to function as an allosteric regulator. It is the second solved structure of 14-3-3 protein complex with the fully active enzyme. Comparison of the 14-3-3:Nth1 complex structure with those of other 14-3-3 complexes highlights the ability of 14-3-3 to modulate the structure and function of many client proteins important for the regulation of key cell processes. This project was funded by Czech Science Foundation (Project P207/11/0455).
Results were published in: Alblova et al. (2017) PNAS; Kopecka et al. (2014) JBC; Macakova et al. (2013) BBA-Gen. Subjects; Veisova et al. (2012) Biochemical Journal.
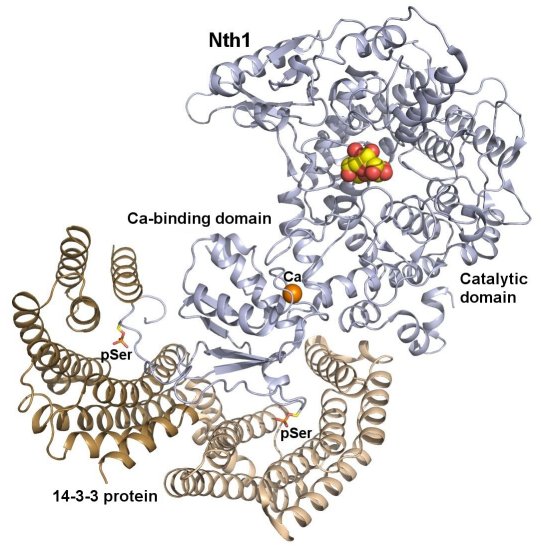
Crystal structure of the complex between phosphorylated Nth and Bmh1 (yeast 14-3-3 protein) (PDB 5N6N). The protomers of the Bmh1 homodimer are shown in yellow and brown. Nth1 is shown in blue. The phosphorylated Ser60 and Ser83 are shown as sticks. The calcium ion is shown in orange. Structural analysis revealed that the binding of phosphorylated Nth1 by 14-3-3 triggers Nth1’s activity by enabling the proper 3D configuration of Nth1’s catalytic and calcium-binding domains relative to each other, thus stabilizing the flexible part of the active site required for catalysis (Alblova et al. (2017) PNAS USA).

Specific trehalase activity of pNth1 WT and mutants upon the Bmh1 activation in vitro (Kopecka et al. (2014) JBC)
Protein kinase ASK1
ASK1 is a member of the mitogen-activated protein kinase kinase kinase family, activates JNK and p38 MAP kinase signaling pathways in response to various stress stimuli, including oxidative stress, endoplasmic reticulum stress, and calcium ion influx . ASK1 plays a key role in the pathogenesis of multiple diseases including cancer, neurodegeneration and cardiovascular diseases and is considered as a promising therapeutic target. The activity of ASK1 is regulated by several other proteins including thioredoxin and the 14-3-3 protein that both function as physiological inhibitors of ASK1. The main goal of this project is to study the role of the 14-3-3 proteins in the regulation of the kinase activity of the catalytic domain of protein kinase ASK1 (Apoptosis signal-regulating kinase 1). The 14-3-3 protein binding to the catalytic domain of ASK1 inhibits ASK1 function through unknown mechanism. To better understand this process we propose: (i) to prepare protein complexes between the catalytic kinase domain of ASK1 and various 14-3-3 protein isoforms; (ii) to determine enzyme kinetic parameters of the kinase domain of ASK1 both in its apo state and in complex with various 14-3-3 protein isoforms; (iii) to map protein-protein interactions within the prepared complexes and to perform their biophysical and structural characterization. Recombinant protein expression, site-directed mutagenesis, methods of enzyme kinetics, analytical ultracentrifugation, mass spectrometry, fluorescence spectroscopy, protein crystallography and SAXS will be used as principal tools. This project was funded by Czech Science Foundation (Project 14-10061S).
Results were published in: Kosek et al. (2014) J. Biol. Chem.; Petrvalska et al. (2016) J. Biol. Chem.; Kylarova et al. (2016) FEBS J.

Superposition of the SAXS envelope with the model of the Ask1-TBD:Trx1 complex (Kosek et al., J Biol Chem 289: 24463, 2014)
Phosducin (Pdc)
Pdc is a highly conserved acidic phosphoprotein that regulates visual signal transduction by modulating the amount of transducin Gtαβγ heterotrimer through competitionwith the Gta subunit for binding to the Gtβγ complex. Pdc was also shown to regulate the cardiovascular system by modulating sympathetic activity and blood pressure. Phosducin function is regulated through the phosphorylation and the binding to the 14-3-3 protein. This project was funded by Czech Science Foundation (Project P305/11/0708).
Results were published in: Rezabkova et al. (2012) Biophys. J.; Kacirova et al. (2015) J. Biol. Chem.; Kacirova et al. (2017) Biophys. J.

Schematic representation of the primary structure of Pdc (Rezabkova et al., Biophys. J 103: 1960-1969).

